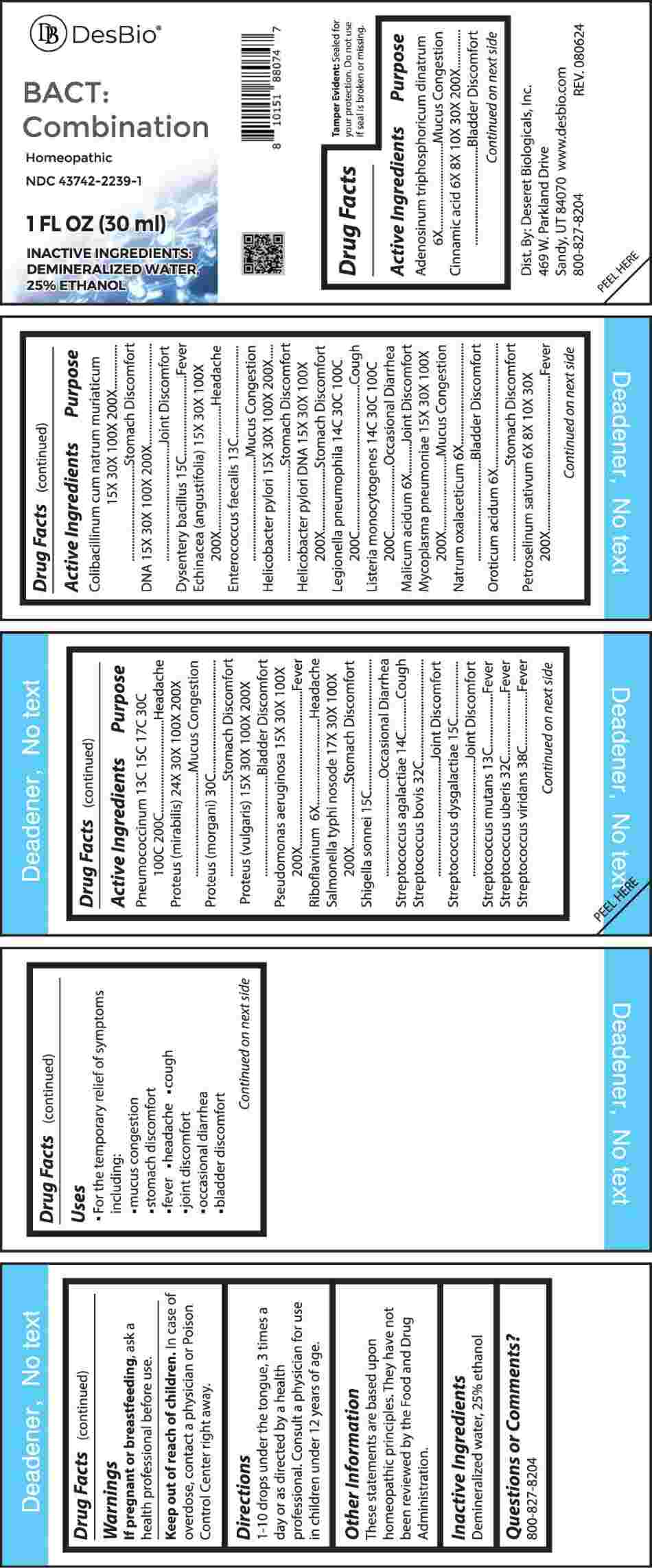
Label: BACT COMBINATION (adenosinum triphosphoricum dinatrum, cinnamic acid, colibacillinum cum natrum muriaticum, streptococcus bovis dna, dysentery bacillus, echinacea (angustifolia), enterococcus faecalis, helicobacter pylori, helicobacter pylori dna, legionella pneumophila, listeria monocytogenes, malicum acidum, mycoplasma pneumoniae, natrum oxalaceticum, oroticum acidum, petroselinum sativum, pneumococcinum, proteus (mirabilis), proteus (morgani), proteus- vulgaris, pseudomonas aeruginosa, riboflavinum, liquid
- NDC Code(s): 43742-2239-1
- Packager: Deseret Biologicals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS:
Adenosinum Triphosphoricum Dinatrum 6X, Cinnamic Acid 6X, 8X, 10X, 30X, 200X, Colibacillinum Cum Natrum Muriaticum 15X, 30X, 100X, 200X, DNA 15X, 30X, 100X, 200X, Dysentery Bacillus 15C, Echinacea (Angustifolia) 15X, 30X, 100X, 200X, Enterococcus Faecalis 13C, Helicobacter Pylori 15X, 30X, 100X, 200X, Helicobacter Pylori DNA 15X, 30X, 100X, 200X, Legionella Pneumophila 14C, 30C, 100C, 200C, Listeria Monocytogenes 14C, 30C, 100C, 200C, Malicum Acidum 6X, Mycoplasma Pneumoniae 15X, 30X, 100X, 200X, Natrum Oxalaceticum 6X, Oroticum Acidum 6X, Petroselinum Sativum 6X, 8X, 10X, 30X, 200X, Pneumococcinum 13C, 15C, 17C, 30C, 100C, 200C, Proteus (Mirabilis) 24X, 30X, 100X, 200X, Proteus (Morgani) 30C, Proteus (Vulgaris) 15X, 30X, 100X, 200X, Pseudomonas Aeruginosa 15X, 30X, 100X, 200X, Riboflavinum 6X, Salmonella Typhi Nosode 17X, 30X, 100X, 200X, Shigella Sonnei 15C, Streptococcus Agalactiae 14C, Streptococcus Bovis 32C, Streptococcus Dysgalactiae 15C, Streptococcus Mutans 13C, Streptococcus Uberis 32C, Streptococcus Viridans 38C.
-
PURPOSE:
Adenosinum Triphosphoricum Dinatrum – Mucus Congestion, Cinnamic Acid – Bladder Discomfort, Colibacillinum Cum Natrum Muriaticum – Stomach Discomfort, DNA – Joint Discomfort, Dysentery Bacillus - Fever, Echinacea (Angustifolia) - Headache, Enterococcus Faecalis – Mucus Congestion, Helicobacter Pylori – Stomach Discomfort, Helicobacter Pylori DNA – Stomach Discomfort, Legionella Pneumophila - Cough, Listeria Monocytogenes – Occasional Diarrhea, Malicum Acidum – Joint Discomfort, Mycoplasma Pneumoniae – Mucus Congestion, Natrum Oxalaceticum – Bladder Discomfort, Oroticum Acidum – Stomach Discomfort, Petroselinum Sativum - Fever, Pneumococcinum - Headache, Proteus (Mirabilis) – Mucus Congestion, Proteus (Morgani) – Stomach Discomfort, Proteus (Vulgaris) – Bladder Discomfort, Pseudomonas Aeruginosa - Fever, Riboflavinum - Headache, Salmonella Typhi Nosode – Stomach Discomfort, Shigella Sonnei – Occasional Diarrhea, Streptococcus Agalactiae - Cough, Streptococcus Bovis – Joint Discomfort, Streptococcus Dysgalactiae – Joint Discomfort, Streptococcus Mutans - Fever, Streptococcus Uberis - Fever, Streptococcus Viridans - Fever
- USES:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
BACT COMBINATION
adenosinum triphosphoricum dinatrum, cinnamic acid, colibacillinum cum natrum muriaticum, streptococcus bovis dna, dysentery bacillus, echinacea (angustifolia), enterococcus faecalis, helicobacter pylori, helicobacter pylori dna, legionella pneumophila, listeria monocytogenes, malicum acidum, mycoplasma pneumoniae, natrum oxalaceticum, oroticum acidum, petroselinum sativum, pneumococcinum, proteus (mirabilis), proteus (morgani), proteus (vulgaris), pseudomonas aeruginosa, riboflavinum, liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43742-2239 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADENOSINE TRIPHOSPHATE DISODIUM (UNII: 5L51B4DR1G) (ADENOSINE TRIPHOSPHATE - UNII:8L70Q75FXE) ADENOSINE TRIPHOSPHATE DISODIUM 6 [hp_X] in 1 mL CINNAMIC ACID (UNII: U14A832J8D) (CINNAMIC ACID - UNII:U14A832J8D) CINNAMIC ACID 6 [hp_X] in 1 mL ESCHERICHIA COLI (UNII: 514B9K0L10) (ESCHERICHIA COLI - UNII:514B9K0L10) ESCHERICHIA COLI 15 [hp_X] in 1 mL HERRING SPERM DNA (UNII: 51FI676N6F) (HERRING SPERM DNA - UNII:51FI676N6F) HERRING SPERM DNA 15 [hp_X] in 1 mL SHIGELLA DYSENTERIAE (UNII: 1EP6R5562J) (SHIGELLA DYSENTERIAE - UNII:1EP6R5562J) SHIGELLA DYSENTERIAE 15 [hp_C] in 1 mL ECHINACEA ANGUSTIFOLIA WHOLE (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA WHOLE 15 [hp_X] in 1 mL ENTEROCOCCUS FAECALIS (UNII: 15E04LZ9CT) (ENTEROCOCCUS FAECALIS - UNII:15E04LZ9CT) ENTEROCOCCUS FAECALIS 13 [hp_C] in 1 mL HELICOBACTER PYLORI (UNII: U09W5JOL3Z) (HELICOBACTER PYLORI - UNII:U09W5JOL3Z) HELICOBACTER PYLORI 15 [hp_X] in 1 mL HELICOBACTER PYLORI DNA (UNII: 14ZY3JP7M6) (HELICOBACTER PYLORI DNA - UNII:14ZY3JP7M6) HELICOBACTER PYLORI DNA 15 [hp_X] in 1 mL LEGIONELLA PNEUMOPHILA (UNII: TJR6ZFY0F0) (LEGIONELLA PNEUMOPHILA - UNII:TJR6ZFY0F0) LEGIONELLA PNEUMOPHILA 14 [hp_C] in 1 mL LISTERIA MONOCYTOGENES (UNII: 3O44K14A86) (LISTERIA MONOCYTOGENES - UNII:3O44K14A86) LISTERIA MONOCYTOGENES 14 [hp_C] in 1 mL MALIC ACID (UNII: 817L1N4CKP) (MALIC ACID - UNII:817L1N4CKP) MALIC ACID 6 [hp_X] in 1 mL MYCOPLASMA PNEUMONIAE (UNII: JQE470FAD0) (MYCOPLASMA PNEUMONIAE - UNII:JQE470FAD0) MYCOPLASMA PNEUMONIAE 15 [hp_X] in 1 mL SODIUM DIETHYL OXALACETATE (UNII: 6CA025Y4FG) (DIETHYL OXALACETATE - UNII:15S56468G7) SODIUM DIETHYL OXALACETATE 6 [hp_X] in 1 mL OROTIC ACID MONOHYDRATE (UNII: 91532S02AO) (OROTIC ACID - UNII:61H4T033E5) OROTIC ACID MONOHYDRATE 6 [hp_X] in 1 mL PETROSELINUM CRISPUM WHOLE (UNII: 1WZA4Y92EX) (CARUM PETROSELINUM (PARSLEY) EXTRACT - UNII:1WZA4Y92EX) PETROSELINUM CRISPUM WHOLE 6 [hp_X] in 1 mL STREPTOCOCCUS PNEUMONIAE (UNII: BT6U234YR2) (STREPTOCOCCUS PNEUMONIAE - UNII:BT6U234YR2) STREPTOCOCCUS PNEUMONIAE 13 [hp_C] in 1 mL PROTEUS MIRABILIS (UNII: C177VR41DV) (PROTEUS MIRABILIS - UNII:C177VR41DV) PROTEUS MIRABILIS 24 [hp_X] in 1 mL PROTEUS MORGANII (UNII: 56X6LID5ZY) (PROTEUS MORGANII - UNII:56X6LID5ZY) PROTEUS MORGANII 30 [hp_C] in 1 mL PROTEUS VULGARIS (UNII: 11T9HCO30O) (PROTEUS VULGARIS - UNII:11T9HCO30O) PROTEUS VULGARIS 15 [hp_X] in 1 mL PSEUDOMONAS AERUGINOSA (UNII: Y793W5V55N) (PSEUDOMONAS AERUGINOSA - UNII:Y793W5V55N) PSEUDOMONAS AERUGINOSA 15 [hp_X] in 1 mL RIBOFLAVIN (UNII: TLM2976OFR) (LACTOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 6 [hp_X] in 1 mL SALMONELLA ENTERICA ENTERICA SEROVAR TYPHI (UNII: 760T5R8B3O) (SALMONELLA ENTERICA ENTERICA SEROVAR TYPHI - UNII:760T5R8B3O) SALMONELLA ENTERICA ENTERICA SEROVAR TYPHI 17 [hp_X] in 1 mL SHIGELLA SONNEI (UNII: OO358E3009) (SHIGELLA SONNEI - UNII:OO358E3009) SHIGELLA SONNEI 15 [hp_C] in 1 mL STREPTOCOCCUS AGALACTIAE (UNII: 2B3763S671) (STREPTOCOCCUS AGALACTIAE - UNII:2B3763S671) STREPTOCOCCUS AGALACTIAE 14 [hp_C] in 1 mL STREPTOCOCCUS EQUINUS (UNII: R2P4NKP3ZK) (STREPTOCOCCUS EQUINUS - UNII:R2P4NKP3ZK) STREPTOCOCCUS EQUINUS 32 [hp_C] in 1 mL STREPTOCOCCUS DYSGALACTIAE (UNII: LN0SH02Y5M) (STREPTOCOCCUS DYSGALACTIAE - UNII:LN0SH02Y5M) STREPTOCOCCUS DYSGALACTIAE 15 [hp_C] in 1 mL STREPTOCOCCUS MUTANS (UNII: 5C7J33MJJ1) (STREPTOCOCCUS MUTANS - UNII:5C7J33MJJ1) STREPTOCOCCUS MUTANS 13 [hp_C] in 1 mL STREPTOCOCCUS UBERIS (UNII: O84V4ZK4BX) (STREPTOCOCCUS UBERIS - UNII:O84V4ZK4BX) STREPTOCOCCUS UBERIS 32 [hp_C] in 1 mL STREPTOCOCCUS VIRIDANS GROUP (UNII: NNV2379HKR) (STREPTOCOCCUS VIRIDANS GROUP - UNII:NNV2379HKR) STREPTOCOCCUS VIRIDANS GROUP 38 [hp_C] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43742-2239-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 11/18/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/18/2024 Labeler - Deseret Biologicals, Inc. (940741853) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(43742-2239) , api manufacture(43742-2239) , label(43742-2239) , pack(43742-2239)