Label: DOXYLAMINE SUCCINATE AND PYRIDOXINE HYDROCHLORIDE tablet, delayed release
- NDC Code(s): 69452-206-20
- Packager: Bionpharma Inc.,
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DOXYLAMINE SUCCINATE AND PYRIDOXINE HYDROCHLORIDE DELAYED-RELEASE TABLETS safely and effectively. See full prescribing information ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDoxylamine succinate and pyridoxine hydrochloride delayed-release tablets are indicated for the treatment of nausea and vomiting of pregnancy in women who do not respond to conservative ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage Information - Initially, take two doxylamine succinate and pyridoxine hydrochloride delayed-release tablets orally at bedtime (Day 1). If this dose adequately controls symptoms the ...
-
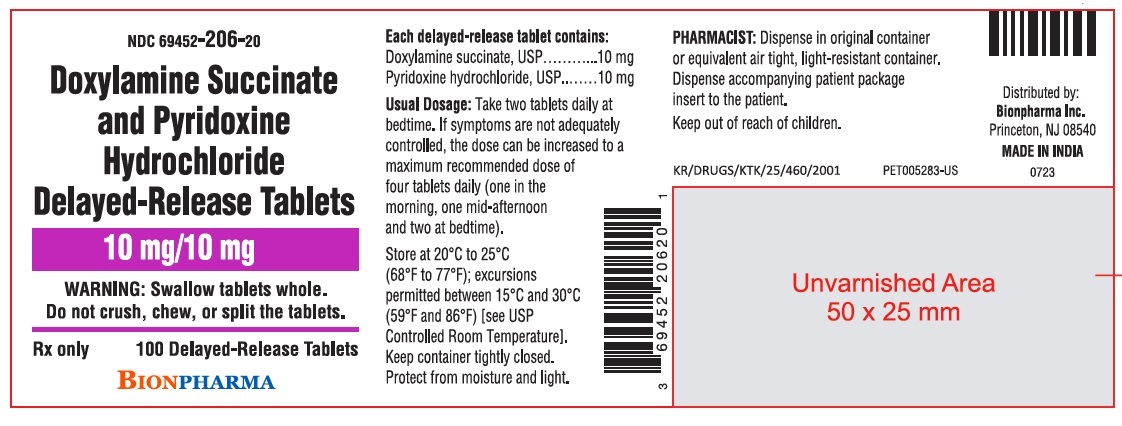
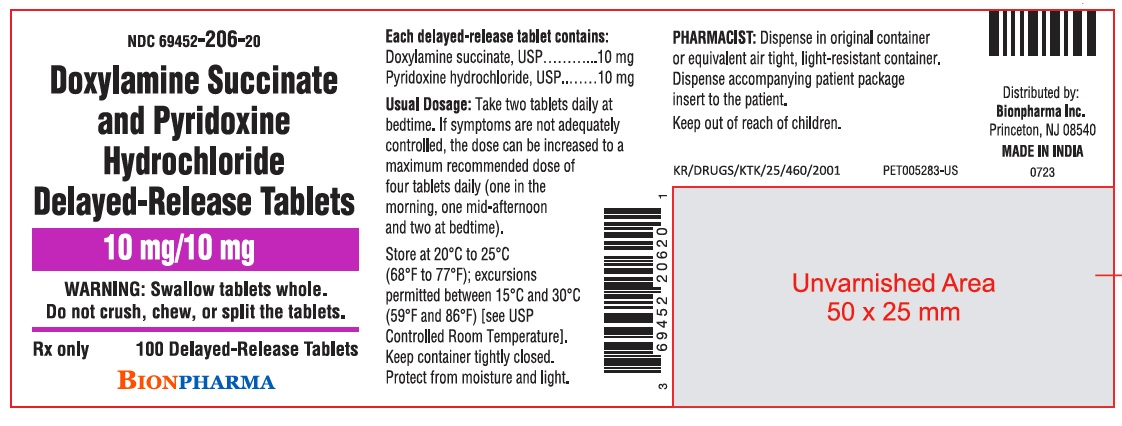
3 DOSAGE FORMS AND STRENGTHSDoxylamine succinate and pyridoxine hydrochloride delayed‑release tablets are white, round, film-coated tablets containing 10 mg doxylamine succinate, USP and 10 mg pyridoxine hydrochloride, USP ...
-
4 CONTRAINDICATIONSDoxylamine succinate and pyridoxine hydrochloride delayed-release tablets are contraindicated in women with any of the following conditions: Known hypersensitivity to doxylamine succinate, other ...
-
5 WARNINGS AND PRECAUTIONS5.1 Activities Requiring Mental Alertness - Doxylamine succinate and pyridoxine hydrochloride may cause somnolence due to the anticholinergic properties of doxylamine succinate, an antihistamine ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed elsewhere in the labeling: Somnolence - [see - Warnings and Precautions (5.1)] Falls or other accidents resulting from the effect of the ...
-
7 DRUG INTERACTIONS7.1 Drug Interactions - Use of doxylamine succinate and pyridoxine hydrochloride is contraindicated in women who are taking monoamine oxidase inhibitors (MAOIs), which prolong and intensify the ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Doxylamine succinate and pyridoxine hydrochloride is intended for the treatment of nausea and vomiting of pregnancy in women who do not respond to conservative ...
-
10 OVERDOSAGE10.1 Signs and Symptoms of Overdose - Doxylamine succinate and pyridoxine hydrochloride is a delayed-release formulation, therefore, signs and symptoms of intoxication may not be apparent ...
-
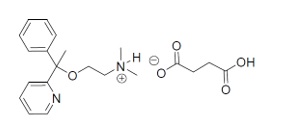
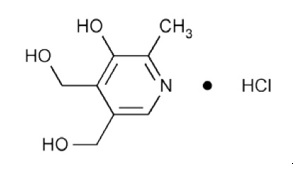
11 DESCRIPTIONDoxylamine succinate and pyridoxine hydrochloride delayed-release tablets are round, white, film‑coated, delayed-release tablets containing 10 mg of doxylamine succinate, USP and 10 mg of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of doxylamine succinate and pyridoxine hydrochloride is unknown. 12.3 Pharmacokinetics - The pharmacokinetics of doxylamine succinate and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity - Two-year carcinogenicity studies in rats and mice have been conducted with doxylamine succinate. Doxylamine succinate ...
-
14 CLINICAL STUDIESA double-blind, randomized, multi-center, placebo-controlled study was conducted to support the safety and efficacy of doxylamine succinate and pyridoxine hydrochloride in the treatment of nausea ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Doxylamine succinate and pyridoxine hydrochloride delayed-release tablets are supplied in a high‑density polyethylene bottle with a polypropylene child-resistant cap and a ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient Information) Somnolence and Severe Drowsiness - Inform women to avoid engaging in activities requiring complete mental alertness, such as driving or ...
-
PATIENT PACKAGE INSERTPatient Information - Doxylamine Succinate and Pyridoxine Hydrochloride - (dox il′ a meen sux′ i nate pir" i dox′ een hye" droe klor′ ide) Delayed-Release Tablets - What are doxylamine ...
-
PRINCIPAL DISPLAY PANELNDC 69452-206-20 - Doxylamine Succinate and Pyridoxine Hydrochloride Delayed-Release Tablets - 10 mg/10 mg - WARNING: Swallow tablets whole. Do not crush, chew, or split the tablets. Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information