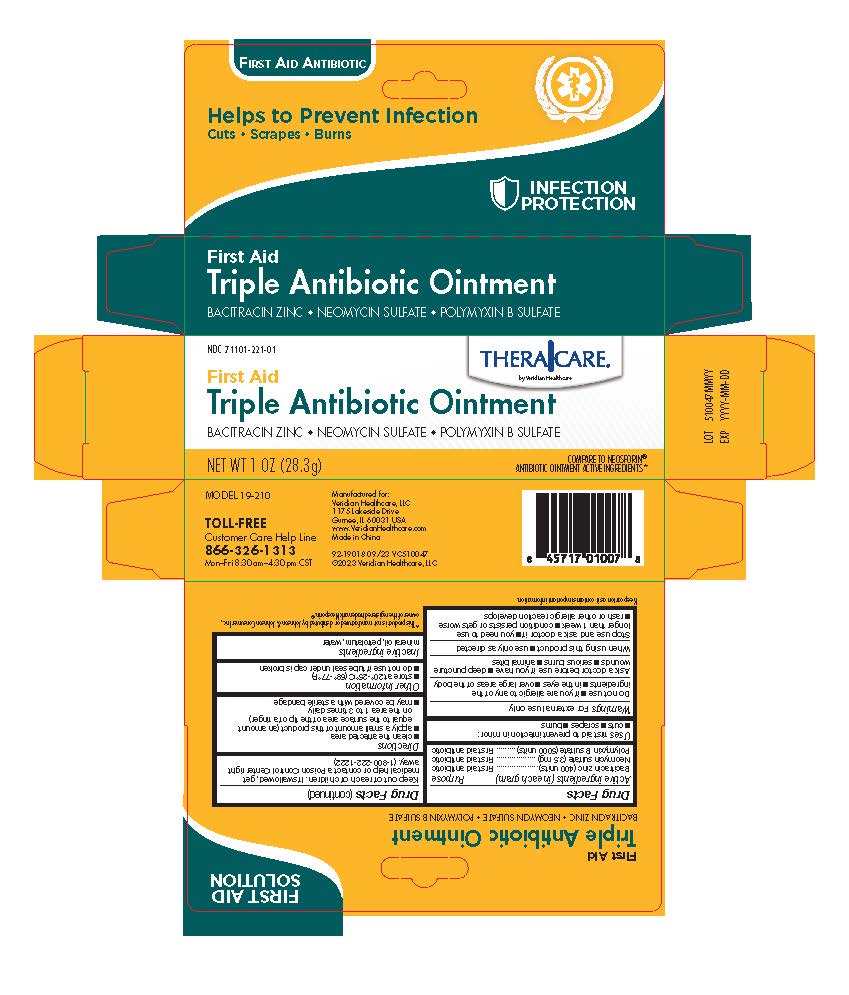
Label: THERACARE TRIPLE ANTIBIOTIC- bacitracin zinc, neomycin sulfate, and polymyxin b sulfate ointment
- NDC Code(s): 71101-221-01
- Packager: Veridian Healthcare
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Use
- Warnings
- Directions
- Other information
- Inactive ingredients
- TheraCare Triple Antibiotic Ointment
-
INGREDIENTS AND APPEARANCE
THERACARE TRIPLE ANTIBIOTIC
bacitracin zinc, neomycin sulfate, and polymyxin b sulfate ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71101-221 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) WATER (UNII: 059QF0KO0R) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71101-221-01 1 in 1 CARTON 12/01/2023 1 28.3 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 12/01/2023 Labeler - Veridian Healthcare (830437997) Establishment Name Address ID/FEI Business Operations Nantong Health & Beyond Hygienic Products Inc. 421280161 manufacture(71101-221)