Label: MACI- autologous cultured chondrocytes implant
-
NDC Code(s):
69866-1030-3,
69866-1030-4,
69866-1030-5,
69866-1030-6, view more69866-1030-7, 69866-1030-8
- Packager: Vericel Corporation
- Category: CELLULAR THERAPY
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated September 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MACI safely and effectively. See full prescribing information for MACI.
MACI® (autologous cultured chondrocytes on porcine collagen membrane)
Cellular sheet for autologous implantation
Initial U.S. Approval: 2016
RECENT MAJOR CHANGES
Dosage and Administration (2.2) 8/2024
INDICATIONS AND USAGE
MACI® is an autologous cellularized scaffold product indicated for the repair of symptomatic, single or multiple full-thickness cartilage defects of the knee with or without bone involvement in adults. (1)
Limitations of Use
- Effectiveness of MACI in joints other than the knee has not been established.
- Safety and effectiveness of MACI in patients over the age of 55 years have not been established.
DOSAGE AND ADMINISTRATION
For autologous implantation only.
- Contact Vericel at 1-800-453-6948 or www.MACI.com regarding training materials for surgical implantation of MACI. (2)
- The amount of MACI implanted depends on the size (surface area in cm2) of the cartilage defect. (2.1)
- MACI should be cut to the size and shape of the defect and implanted with the cell-side down. (2.2)
DOSAGE FORMS AND STRENGTHS
Each 3 x 5 cm cellular sheet (MACI implant) consists of autologous cultured chondrocytes on a resorbable porcine Type I/III collagen membrane, at a density of at least 500,000 cells per cm2. (3)
CONTRAINDICATIONS
- Known history of hypersensitivity to gentamicin, other aminoglycosides, or products of porcine or bovine origin. (4)
- Severe osteoarthritis of the knee. (4)
- Inflammatory arthritis, inflammatory joint disease, or uncorrected congenital blood coagulation disorders. (4)
- Prior knee surgery (within 6 months), excluding surgery to procure a biopsy or a concomitant procedure to prepare the knee for a MACI implant. (4)
- Inability to cooperate with a physician-prescribed post-surgical rehabilitation program. (4)
WARNINGS AND PRECAUTIONS
- Malignancy: The risk of malignancy in the area of cartilage biopsy or implant is unknown. Expansion of malignant or dysplastic cells present in biopsy tissue during manufacture and subsequent implantation may be possible. (5.1)
- Transmissible infectious diseases: Because patients undergoing procedures associated with MACI are not routinely tested for transmissible infectious diseases, cartilage biopsy and MACI implant may carry risk of transmitting infectious diseases. (5.2)
- Presurgical Comorbidities: Local inflammation or active infection in the bone, joint, and surrounding soft tissue, meniscal pathology, cruciate ligament instability, and misalignment should be assessed and treated prior to or concurrent with MACI implantation. (5.3)
- Product Sterility: Final sterility test results are not available at the time of shipping. (5.4)
ADVERSE REACTIONS
The most frequently occurring adverse reactions (≥5%) reported for MACI were arthralgia, tendonitis, back pain, joint swelling, and joint effusion. (6)
Serious adverse reactions reported for MACI were arthralgia, cartilage injury, meniscus injury, treatment failure, and osteoarthritis. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Vericel at 1-800-453-6948 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch for voluntary reporting of adverse reactions.
USE IN SPECIFIC POPULATIONS
Pregnancy: Because MACI implantation requires invasive surgical procedures, use in pregnancy is not recommended. (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Preparation and Implantation Procedure
2.3 Postsurgical Rehabilitation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Malignancy
5.2 Transmissible Infectious Diseases
5.3 Presurgical Assessment of Comorbidities
5.4 Product Sterility
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
MACI® (autologous cultured chondrocytes on porcine collagen membrane) is an autologous cellularized scaffold product indicated for the repair of single or multiple symptomatic, full-thickness cartilage defects of the knee with or without bone involvement in adults.
Limitations of Use
- Effectiveness of MACI in joints other than the knee has not been established.
- Safety and effectiveness of MACI in patients over the age of 55 years have not been established.
-
2 DOSAGE AND ADMINISTRATION
For Autologous Implantation Only.
Contact Vericel at 1-800-453-6948 or www.MACI.com regarding training materials for surgical implantation of MACI.
2.1 Dosage
- The amount of MACI implanted depends on the size (surface area in cm2) of the cartilage defect. The surgeon should cut the MACI implant to the size and shape of the defect, to ensure the damaged area is completely covered.
- MACI implant is for single-use.
- Multiple implants may be used if there is more than one defect. The size of MACI is adjusted for the size of each cartilage defect.
2.2 Preparation and Implantation Procedure
Collection of Autologous Cartilage Biopsy
- The procedure may be performed arthroscopically.
- Using a ring curette or curved notchplasty gouge, harvest at least two (2) healthy full-thickness cartilage specimens from a lesser load-bearing area of the damaged knee, such as the lateral intercondylar notch, the superior lateral trochlear ridge, or the superior medial trochlear ridge.
-
The specimens should measure approximately 5 x 8 mm each (200-300 mg total).
-
The biopsy must be full-thickness and should include a small amount of subchondral bone, which will be removed prior to processing the biopsy.
- Some punctate bleeding may occur at the site of biopsy harvest.
-
Using sterile technique, place the biopsy into transport medium bottle.
Pre-Operative Preparation
- Confirm that the patient’s identity matches the patient identifiers on the MACI labels.
- Inspect the sealed MACI shipping box for any evidence of damage.
- Open the MACI shipping box and inspect the internal packaging for leaks (liquid) in the outer bag or self-seal pouch containing the bottle holding the MACI implant or for any evidence of damage or contamination.
- DO NOT USE if the patient identifiers do not match, or there are signs of leaking or damage to the packaging. Contact MACI representative immediately or call Vericel Customer Care at 1-800-453-6948.
- After inspection, keep MACI in its original packaging and store at room temperature until the surgical site has been prepared.
- Arthroscopic delivery is for lesions that are a maximum of 4 cm2 in size and are accessible using an arthroscopic approach.
Implantation Procedure
- Perform implantation procedure during arthrotomy or arthroscopy using sterile surgical techniques.
- Follow the implantation with an appropriate, physician-prescribed rehabilitation program [see Dosage and Administration (2.3)].
- NOTE:
- The MACI Surgical Implantation Kit may be used to assist with MACI knee surgery via arthrotomy.
- The MACI Arthroscopic Instruments may be used to assist with arthroscopic delivery.
-
Ensure instruments selected are appropriately matched to the defect size.
Preparing Defect
Arthroscopic Delivery:
- Create an appropriately placed portal to to visualize the defect to be treated athroscopically.
- Under direct arthroscopic vision insert a spinal needle directly over the center of the cartilage defect.
- Use the location determined by the spinal needle to create a second portal for the cannula (e.g., MACI Cannula Assembly) directly perpendicular to the defect.
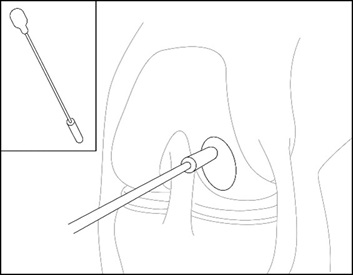
- Measure the length and width of the defect using a measurement probe (e.g., MACI Arthroscopic Measurement Probe) as shown in Figure 1.
Figure 1: Measure defect size – Arthroscopic Method
- Select and insert the appropriate size cannula (e.g., MACI Cannula Assembly) in this second portal directly over the center of the cartilage defect.
- Remove trocar from cannula assembly.
- Select the appropriate size cutter (e.g., MACI Arthroscopic Cutter) based on the defect size.
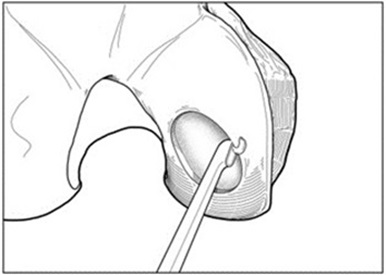
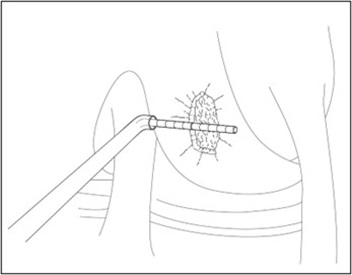
- Insert the cutter through the cannula and position over the cartilage defect. Adjust the angle of the cutter head, and apply pressure to score the cartilage surrounding the defect (Figure 2). Bring the cutter head back to its original position prior to removal from the joint.
Figure 2: Score Cartilage – Arthroscopic Method
For Arthroscopic and Arthrotomy Delivery:
-
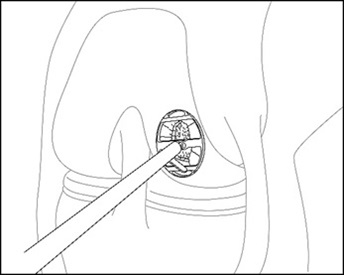
For chondral defects, remove all damaged and fibrous tissue on the defect bed using appropriate instruments (e.g., MACI Rake Curette, MACI Square Curette and MACI Open-Ring Curette). Debride the defect bed back to stable cartilage with vertical walls down to the subchondral bone by removing as little healthy cartilage as possible (Figure 3). Do not penetrate the subchondral bone.
- For osteochondral defects, debride the defect bed back to stable cartilage with vertical walls down to healthy stable bone.
- Avoid bleeding through the subchondral plate. If bleeding occurs, use a suitable hemostatic agent to control the bleeding.
Preparing MACI Implant
- Unpacking MACI implant box (outside sterile field).
- Unpack MACI implant shipping box.
- Staff responsible for removing bottle from packaging and decanting contents into sterile container in sterile field should wear protective gown, gloves, and eyewear.
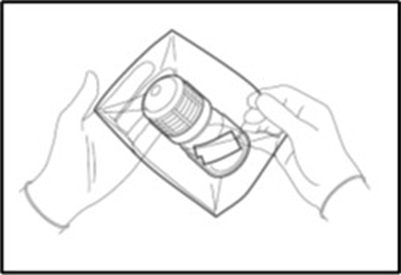
- Remove the outer bag containing a bottle holding the MACI implant.
- Remove the self-seal pouch containing the bottle from the outer bag (Figure 4).
- Tear notches on the self-seal pouch to open the pouch and remove the bottle.
Note: Keep the bottle upright and do not open the cap until ready to use the MACI implant.
Note: Do not remove the MACI implant from the bottle until ready to be used.
- Unpacking the MACI implant bottle (Figure 5)
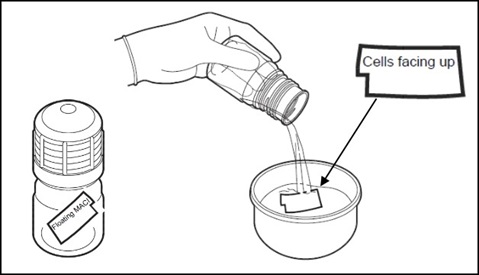
- When ready, a team member outside the sterile field but adjacent to the sterile prep table, will twist open and remove the cap from the bottle. The outside of the bottle is not sterile, ensure that the MACI implant or sterile instruments do not come in contact with the outside of the bottle.
- On sterile field, place sterile dish or container of sufficient size to hold contents of MACI implant bottle, approximately 120 mL of media.
- Ensure MACI implant is free floating and not adhered to inner surface of bottle.
Aseptically and rapidly decant the bottle contents into sterile dish including all transport media and the MACI implant.
Note: If the MACI implant remains in the bottle, media from the sterile dish can be returned to the bottle via sterile syringe and decanted again. Alternatively, if the MACI has remained in the neck of the bottle, it can be extracted using sterile non-toothed forceps to grasp an edge or corner.
Figure 5: Unpacking MACI Implant - The MACI implant has a rough side and a smooth side. The cells are seeded on the rough side. A notch in the lower left corner of the implant in landscape orientation indicates that the cell-side is facing up.
- The cell-side of the MACI implant should remain facing up at all times until placement into the defect. If the MACI implant is cell-side down after decanting from bottle, a sterile field team member will flip the membrane using two (2) sterile non-toothed forceps. The MACI implant should only be grasped by corners or edges.
-
Sterile field team member will use two (2) sterile non-toothed forceps to grasp the MACI implant corners and place the MACI implant cell-side up onto a sterile work surface (e.g., MACI Cutting Block or MACI Arthroscopic Cutting Block). A notch in the lower left corner of the implant indicates that the cell-side is facing up.
Note: The MACI implant must remain hydrated with the shipping media.
Use the decanted media from the sterile dish to maintain implant hydration after removal from the bottle.Shaping the MACI implant
- Avoid excessive manipulation of the MACI implant. Throughout the shaping and delivery of the MACI implant it is important to keep track of membrane orientation to facilitate placing cells in the base of the defect.
Arthroscopic Delivery:
-
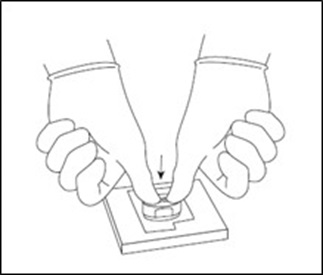
Using the MACI Cutter that was packaged together with the Arthroscopic Cutter, place the MACI Cutter on top of the MACI implant and use light thumb pressure or lightly tap with a mallet to cut to size (Figure 6).
Figure 6: Cut MACI Implant
- Place any remaining MACI implant into a separate intermediary dish with adequate media to keep the implant hydrated.
Arthrotomy Delivery:
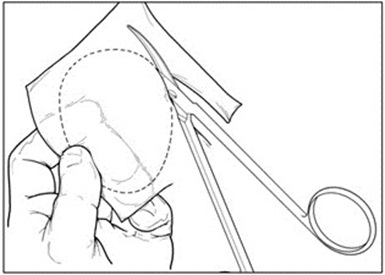
- Create an exact template of the defect (Figure 7).
Figure 7: Creating Defect Template
- Create orientation markers on the template to assist with proper orientation of the MACI implant. Turn the marked template over to ensure that the cells will be properly placed into the defect.
- To maintain proper orientation, turn the template over and place it underneath the MACI implant, against the smooth, non-seeded side. The template should be visible through the translucent implant.
Note: Ensure minimal contact with the cell-seeded surface of the MACI implant.
- Using the template as a guide, cut the MACI implant to the correct size and shape.
- Place the custom-cut implant into a sterile intermediary dish, ensuring the cell-side up orientation. Ensure MACI implant remains hydrated at all times. Using sterile syringe, hydrate implant using decanted media.
- Place any remaining MACI implant into a separate intermediary dish with adequate media to keep the implant hydrated.
Placing MACI Implant
Arthroscopic Delivery
- Turn off fluid and drain the knee.
-
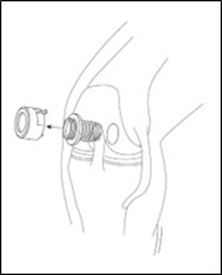
If using a cannula with a dam (e.g., MACI Arthroscopic instrument), remove the dam from the cannula (Figure 8). Do not insert the MACI implant through a cannula dam.
Figure 8: Remove Cannula Dam
-
A dry arthroscopic environment is essential for implantation. Remove any close liquid collections with the suction tip and ensure the defect area is dry and free of bleeding (e.g., using the spongy end of the MACI Applicator) (Figure 9).
Figure 9: Dry Defect
-
Select appropriate size MACI V-Shuttle Delivery Device that matches the MACI Cannula diameter size used.
-
Verify the V-Shuttle delivery device functionality by pressing the plunger to deploy the antennae prior to use.
-
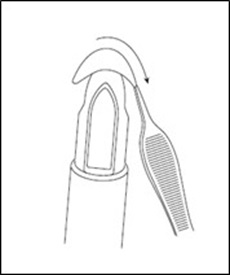
Gently place the MACI implant cell-side (rough side) up on the tip of the MACI V-Shuttle delivery device using forceps (Figure 10). Maintain the orientation of the V-Shuttle in an upright position.
Figure 10: Placement on MACI V-Shuttle Delivery Device
-
Apply a thin layer of fibrin sealant to the entire base of the defect (bone) bed.
-
Insert the MACI V-Shuttle with the loaded MACI implant into the MACI cannula ensuring the outer fins of the V Shuttle are oriented inferior and superior in line with the lesion. Slowly slide V-Shuttle through the cannula until the implant is positioned just over the defect.
-
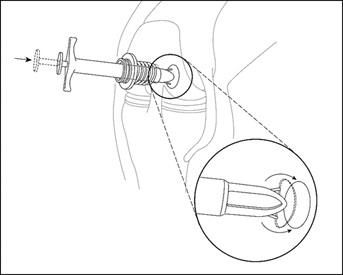
Once the MACI implant and MACI V-Shuttle are properly positioned directly over the center of the defect, gently push the plunger on the V-Shuttle to deploy the antennae to deliver the implant into the defect (Figure 11).
Note: Do not compress the implant between the bone and the tip of the V-Shuttle. This may damage the cells on the MACI implant.
-
Once the MACI implant is delivered into the defect, depress the plunger to retract the antennae and slowly retract the V-Shuttle from the cannula.
Figure 11: Arthroscopic Delivery of MACI Implant to Defect
-
Gently adjust placement of the MACI implant to desired location using a tamp (e.g., the silicone end of the MACI Applicator) and allow at least 3 minutes for the sealant to set (Figure 12).
Figure 12: Placement of the MACI Implant
- Assess that the fibrin sealant has set and the MACI implant has adhered through the following methods: gently probing the implant with a blunt instrument (e.g. MACI Applicator), and repeated cycles of flexion and extenson of the knee.
Arthrotomy Delivery:
- Ensure defect area is dry and free of bleeding.
- Apply a thin layer of fibrin sealant to the entire base of the defect (bone) bed.
- Maintaining appropriate rotational orientation, place the custom-cut implant onto the defect bed cell-side down.
- Apply light digital pressure to the implant for approximately 3 minutes to allow sealant to set.
-
Assess that the fibrin sealant has set and the MACI implant has adhered through the following methods: gently probing the implant with a blunt instrument (e.g. MACI Applicator), and repeated cycles of flexion and extension of the knee.
-
MACI implant fixation may also be supplemented with interrupted resorbable sutures if desired or if conditions warrant, particularly if the defect is uncontained (i.e, the cartilage defect is not 100% surrounded by a stable cartilage rim) or the lesion is larger than 10 cm2.
For Arthroscopic and Arthrotomy Delivery:
- Fibrin sealant may also be applied to the rim (periphery) of the implant.
- Discard unused MACI implant and media using bio-hazard hospital protocols.
2.3 Postsurgical Rehabilitation
A physician-prescribed rehabilitation program that includes early mobilization, joint range of motion, and weight bearing is recommended to promote graft maturation and reduce the risk of graft delamination, postoperative thromboembolic events, and joint stiffness. Stage this program to promote a progressive return to full joint range of motion and weight-bearing as well as muscle strengthening and conditioning. Return to recreational and sporting activity should be in consultation with healthcare professionals.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
MACI is contraindicated in patients with the following conditions:
- Known history of hypersensitivity to gentamicin, other aminoglycosides, or products of porcine or bovine origin. [see Description (11)]
- Severe osteoarthritis of the knee (Kellgren-Lawrence grade 3 or 4).
- Inflammatory arthritis, inflammatory joint disease, or uncorrected congenital blood coagulation disorders.
- Prior knee surgery (6 months), excluding surgery to procure a biopsy or a concomitant procedure to prepare the knee for a MACI implant.
- Inability to cooperate with a physician-prescribed post-surgical rehabilitation program [See Dosage and Administration (2.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Malignancy
The safety of MACI used in patients with malignancy in the area of cartilage biopsy or implant is unknown. The potential exists for expansion of malignant or dysplastic cells present in biopsy tissue during manufacture and subsequent implantation. In addition, implantation of normal autologous chondrocytes could theoretically stimulate growth of malignant cells in the area of the implant, although there have been no such incidents reported in humans or animals.
5.2 Transmissible Infectious Diseases
MACI is intended solely for autologous use. Patients undergoing the surgical procedures associated with MACI are not routinely tested for transmissible infectious diseases. Therefore, the cartilage biopsy and the MACI implant may carry the risk of transmitting infectious diseases to personnel handling these tissues. Accordingly, healthcare providers should employ universal precautions in handling the biopsy samples and the MACI product.
Product manufacture includes reagents derived from animal materials. All animal-derived reagents are tested for viruses, retroviruses, bacteria, fungi, yeast, and mycoplasma before use. Bovine materials are sourced to minimize the risk of transmitting a prion protein that causes bovine spongiform encephalopathy and may cause a rare fatal condition in humans called variant Creutzfeldt-Jakob disease.
These measures do not totally eliminate the risk of transmitting these or other transmissible infectious diseases and disease agents. Report the occurrence of a transmitted infection to Vericel Corporation at 1-800-453-6948.
5.3 Presurgical Assessment of Comorbidities
To create a favorable environment for healing, assess and treat the following conditions prior to or concurrent with implantation with MACI:
- Local inflammation or active infection in the bone, joint, and surrounding soft tissue: patients should be deferred until complete recovery.
- Meniscal pathology: presence of an unstable or torn meniscus requires partial resection, repair, or replacement prior to or concurrent with MACI implantation. MACI is not recommended in patients with a total meniscectomy.
- Cruciate ligament instability: the joint should not possess excessive laxity, which may create excessive shear and rotational forces across the joint. Both anterior and posterior cruciate ligaments should be stable or undergo reconstruction prior to or concurrent with MACI implantation.
- Misalignment: the tibio-femoral joint should be properly aligned, and patella tracking should be normalized. Varus or valgus misalignment of the tibio-femoral joint and abnormal patella tracking may abnormally load joint surfaces and jeopardize the implant. Misalignment and patella tracking should be addressed with a corrective osteotomy or similar corrective procedure prior to or concurrent with MACI implantation.
5.4 Product Sterility
MACI is shipped after passing preliminary test results from in-process microbial tests. A final sterility test is initiated prior to shipping, but the result will not be available prior to implantation. If microbial contamination is detected after the product has been shipped, Vericel will notify the healthcare provider(s) and recommend appropriate actions.
-
6 ADVERSE REACTIONS
The most frequently occurring adverse reactions (≥5%) reported for MACI were arthralgia, tendonitis, back pain, joint swelling, and joint effusion.
Serious adverse reactions reported for MACI were arthralgia, cartilage injury, meniscus injury, treatment failure, and osteoarthritis.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a product cannot be directly compared to rates in the clinical trials of another product and may not reflect the rates observed in practice.
In a 2-year prospective, multicenter, randomized, open-label, parallel-group clinical trial, 144 patients, ages 18 to 54 years, were randomized to receive a 1-time treatment with MACI or microfracture (1:1, 72 patients in each treatment group). Demographic characteristics of patients in the trial were similar in both treatment groups. The majority of patients were male (62.5% MACI, 66.7% microfracture), and the mean ages were 34.8 (MACI) and 32.9 (microfracture) years. Overall, 70 patients in the MACI group and 67 patients in the microfracture group completed 2 years of follow-up.
In addition, all 144 subjects from the 2-year clinical trial had the option to enroll in a 3-year follow-up study (extension study). Safety and efficacy assessments were performed at yearly scheduled visits. The demographic characteristics of patients (N = 128) enrolled in the extension study were similar in both treatment groups and consistent with the overall population of the 2-year clinical trial.
The proportion of patients with at least one (1) subsequent surgical procedure (any surgical procedure performed on the treated knee joint, including arthroscopy, arthrotomy, or manipulation under anesthesia) in the 2 years following study treatment was comparable between treatment groups (8.3% in the MACI group and 9.7% in the microfracture group).
Adverse reactions reported in ≥5% of patients in either treatment group in the 2-year clinical trial are provided in Table 1.
Table 1. Adverse Reactions in ≥5% of Patients in Any Treatment Group in the 2-Year Clinical Trial System Organ Class MACI
n = 72
n (%)Microfracture
n = 72
n (%)Musculoskeletal and Connective Tissue Disorders Arthralgia 37 (51.4) 46 (63.9) Back pain 8 (11.1) 7 (9.7) Joint swelling 7 (9.7) 4 (5.6) Joint effusion 5 (6.9) 4 (5.6) Injury, Poisoning and Procedural Complications Cartilage injury 3 (4.2) 9 (12.5) Ligament sprain 3 (4.2) 5 (6.9) Procedural pain 3 (4.2) 4 (5.6) General Disorders and Administration Site Conditions Treatment failure 1 (1.4) 4 (5.6) In the 3-year extension study, adverse reactions reported in ≥5% of patients were (MACI vs microfracture): arthralgia (46.2% vs 50.8%), tendonitis (6.2% vs 1.6%), back pain (4.6% vs 6.3%), osteoarthritis (4.6% vs 7.9%), joint effusion (3.1% vs 7.9%), cartilage injury (6.2% vs 15.9%), procedural pain (3.1% vs 7.9%), ligament sprain (1.5% vs 7.9%), and treatment failure (4.6% vs 7.9%).
Serious adverse reactions reported in patients in either treatment group for integrated data across the 2-year clinical trial and the 3-year extension study are provided in Table 2.
Table 2.Serious Adverse Reactions in Patients in Any Treatment Group Across the 2-Year Clinical Trial and the 3-Year Extension Study System Organ Class MACI
n = 72
n (%)Microfracture
n = 72
n (%)Musculoskeletal and Connective Tissue Disorders Arthralgia 1 (1.4) 7 (9.7) Back pain 0 3 (4.2) Joint swelling 3 (4.2) 0 Joint effusion 3 (4.2) 0 Injury, Poisoning and Procedural Complications Cartilage injury 3 (4.2) 8 (11.1) General Disorders and Administration Site Conditions Treatment failure 3 (4.2) 7 (9.7) 6.2 Postmarketing Experience
Graft complication (e.g., abnormalities to the repair graft that become symptomatic; this could include graft overgrowth [tissue hypertrophy], under-fill or damage to the repair tissue that has elicited a painful response, or mechanical symptoms), graft delamination (i.e., a dislodging of the repair graft from the underlying subchondral bone that has become symptomatic; this can be measured as marginal, partial, or a complete delaminated graft), and tendonitis have been reported during use of MACI outside the United States. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to MACI exposure.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
MACI implantation requires invasive surgical procedures; therefore use during pregnancy is not recommended. Limited clinical data on patients exposed to MACI during pregnancy are available. There are insufficient data with MACI use in pregnant women to inform a product-associated risk. Animal reproduction studies have not been conducted with MACI. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of MACI in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for MACI and any potential adverse effects on the breastfed infant from MACI or from the underlying maternal condition.
-
11 DESCRIPTION
MACI, autologous cultured chondrocytes on porcine collagen membrane, is a cellular sheet that consists of autologous chondrocytes seeded on a 3 x 5 cm, resorbable porcine Type I/III collagen membrane, for implantation into cartilage defects of the knee. The active ingredients of MACI are the autologous cultured chondrocytes and porcine Type I/III collagen. The autologous chondrocytes are propagated in cell culture and are seeded on the collagen at a density of 500,000 to 1,000,000 cells per cm2. The final MACI implant contains at least 500,000 cells per cm2 and does not contain any preservative.
The product manufacture also uses reagents derived from animal materials. The resorbable, Type I/III, collagen membrane, which is a component of MACI, is porcine-derived. Fetal bovine serum is a component in the culture medium used to propagate the autologous chondrocytes; therefore, trace quantities of bovine-derived proteins may be present in MACI. These animal-derived reagents are tested for viruses, retroviruses, bacteria, fungi, yeast, and mycoplasma before use.
MACI may contain residual gentamicin because it is included during manufacture. Gentamicin is not included in the transport medium used to maintain product stability. Studies determined an average of 9.2 μg residual gentamicin per MACI implant.
A final sterility test is initiated prior to shipping, but the result will not be available prior to implantation. Passing results from preliminary in-process microbial tests are required for release of MACI for shipping.
- 12 CLINICAL PHARMACOLOGY
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies to evaluate the carcinogenicity or impairment of fertility potential of MACI have not been performed. In vitro studies have shown that the expansion process for chondrocytes does not induce changes to the cellular karyotype.
Four studies (in vitro and in vivo) were conducted to assess the genotoxic potential of the collagen membrane. The results from these studies demonstrated that the collagen membrane was non-mutagenic.
13.2 Animal Toxicology and/or Pharmacology
Implantation of analogous products in critical-size defects in the hind limbs of rabbits and horses did not reveal any serious safety concerns. The products consisted of the same membrane as MACI with rabbit or horse cells, respectively. Non-clinical testing has shown that the collagen membrane is not toxic and is compatible with biological tissue.
-
14 CLINICAL STUDIES
The effectiveness of MACI implant was evaluated in a 2-year prospective, multicenter, randomized, open-label, parallel-group study, SUMMIT (Superiority of MACI implant versus Microfracture Treatment in patients with symptomatic articular cartilage defects in the knee), which enrolled a total of 144 subjects, ages 18 to 54 years, with at least one symptomatic Outerbridge Grade III or IV focal cartilage defect on the medial femoral condyle, lateral femoral condyle, and/or the trochlea. Failure of a prior cartilage surgery was not required for study entry. The subjects were randomized to receive either a 1-time treatment with MACI or microfracture. The co-primary efficacy endpoint was change from baseline to Week 104 for the subject’s Knee injury and Osteoarthritis Outcome Score (KOOS) in two subscales: Pain and Function (Sports and Recreational Activities [SRA])¹. Safety also was evaluated through Week 104 [see Adverse Reactions (6.1)].
Of the 72 subjects randomized to MACI, 70 completed the study and two (2) discontinued prematurely (one (1) due to an adverse event [AE] and one (1) wished to withdraw). Of the 72 subjects randomized to microfracture, 67 completed the study and five (5) discontinued prematurely (one (1) due to an AE, one (1) wished to withdraw, and three (3) due to lack of clinical benefit).
At Week 104, KOOS pain and function (SRA) had improved from baseline in both treatment groups, but the improvement was statistically significantly (p = 0.001) greater in the MACI group compared with the microfracture group (Table 3).
Table 3. Change in KOOS Pain and Function (SRA) Scores in the 2-Year Study LS = least squares; KOOS = Knee injury and Osteoarthritis Outcome Score; SD = standard deviation; SRA = Sports and Recreational Activities.
MACI
Mean (SD)Microfracture
Mean (SD)N Pain Function N Pain Function Baseline 72 37.0 (13.5) 14.9 (14.7) 71 35.4 (12.1) 12.6 (16.7) Week 104 72 82.4 (16.2) 60.9 (27.8) 70 70.9 (24.2) 48.7 (30.3) Change From Baseline to Week 104 72 45.4 (21.1) 46.0 (28.4) 69 35.2 (23.9) 35.8 (31.6) LS Means (Week 104) 44.1 46.1 32.4 34.6 Difference * [MACI – Microfracture] 11.8 11.4 p-value † 0.001 In a responder analysis, the proportion of subjects with at least a 10-point improvement in both KOOS pain and function (SRA) was greater in the MACI group (63/72=87.5%; 95% CI [77.6%, 94.1%]) compared with the microfracture group (49/72=68.1%; 95% CI [56.0%, 78.6%]).
All subjects from the 2-year study had the option to enroll in a 3-year follow-up study (extension study), in which 128 subjects participated. All 65 subjects (100%, 65/65) in the MACI group and 59 subjects (93.7%, 59/63) in the microfracture group completed the extension study. The mean 2-year KOOS pain and function scores remained stable for the additional 3-year period in both treatment groups (Table 4).
Table 4. KOOS Pain and Function (SRA) Scores in the 3-Year Extension Study Visit MACI Microfracture N Pain
mean (SD)Function
mean (SD)N Pain
mean (SD)Function
mean (SD)Baseline 65/65 37.1 (13.1) 15.4 (14.8) 63/63 35.2 (12.3) 11.9 (16.2) 2 Years 63/63 82.2 (15.8) 60.5 (26.5) 60/60 71.8 (23.9) 48.9 (30.6) 5 Years 65/64 82.2 (20.1) 61.9 (30.9) 59/59 74.8 (21.7) 50.3 (32.3) - 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
- A single patient order may contain one (1) or two (2) implants, each in its own bottle and shipper, depending on lesion size and number of lesions.
MACI - One (1) Implant
- MACI, NDC69866-1030-5 (outer box), contains one (1) implant supplied ready for use as a single cellular sheet approximately 3 x 5 cm, in a sterile, sealed, translucent perfluoroalkoxy (PFA) resin bottle and cap. Each bottle contains one 3 x 5 cm implant with a 0.5-cm2 section removed from the lower left-hand corner.
MACI - Two (2) Implant
- MACI, NDC69866-1030-8 (outer box), contains two (2) implants supplied ready for use as cellular sheets approximately 3 x 5 cm, in a sterile, sealed, translucent perfluoroalkoxy (PFA) resin bottle and cap. Each bottle contains one 3 x 5 cm implant with a 0.5 cm2 section removed from the lower left-hand corner.
- Each bottle is individually sealed in a clear self-seal pouch. Each self-seal pouch is placed into a 95kPa outer bag with absorbent material. These bags are enclosed in an outer box insulated with ambient temperature gel packs.
Storage and Handling
- Store MACI at room temperature in its original packaging (outer box) until ready to use.
- DO NOT REFRIGERATE or FREEZE, or sterilize MACI.
- DO NOT USE if the bottle is damaged, has been compromised, or has leaked.
- Use MACI prior to 11:59 PM EST on the date of expiration printed on the package.
- Dispose of unused MACI or waste material as surgical biohazardous waste in accordance with local requirements.
- A single patient order may contain one (1) or two (2) implants, each in its own bottle and shipper, depending on lesion size and number of lesions.
-
17 PATIENT COUNSELING INFORMATION
- Advise the patient that:
- A cartilage biopsy is needed to manufacture MACI. The biopsy is typically performed as an arthroscopic procedure at the time of diagnosis confirmation.
- The length of time between the biopsy and the implantation of MACI may vary depending on many factors, including the quality and number of cells obtained from the biopsy. On average this will take 6 weeks; however, cells can be held in storage until a convenient date for surgery is agreed upon between the patient and the surgeon.
- Even if the surgeon has taken a biopsy needed to produce MACI, it may be possible that the patient cannot be treated with MACI, (e.g., in case the biopsy is of insufficient quality to produce MACI, if the cells cannot be grown in the laboratory, or if the expanded cells do not meet all the quality requirements).
- Advise the patient on the risk of graft complications, subsequent surgical procedures, and treatment failure. [See Adverse Reactions (6)]
- Advise the patient on general complications related to knee surgery, which may include deep vein thrombosis and pulmonary embolism.
- Advise the patient to closely follow the physician-prescribed rehabilitation program, which will include limitations and allowances for beginning specific physical activities. [See Dosage and Administration (2.3)]
- Inform patient about the possible risk of transmission of infectious diseases with MACI implantation. [See Warnings and Precautions (5.2)]
Manufactured by: Vericel Corporation, 64 Sidney Street, Cambridge, MA 02139
MACI® is a registered trademark of Vericel Corporation.
Patents: www.vcel.com/research-and-development
© 2024 Vericel Corporation.
65628 Revision 3
08/2024
- Advise the patient that:
- PRINCIPAL DISPLAY PANEL - NDC: 69866-1030-3 - Bottle Label
- PRINCIPAL DISPLAY PANEL - NDC: 69866-1030-4 - Outer Pouch Label
- PRINCIPAL DISPLAY PANEL - NDC: 69866-1030-5 - Shipper Label
- PRINCIPAL DISPLAY PANEL - NDC: 69866-1030-6 - Bottle Label
- PRINCIPAL DISPLAY PANEL - NDC: 69866-1030-7 - Outer Pouch Label
- PRINCIPAL DISPLAY PANEL - NDC: 69866-1030-8 - Shipper Label
-
INGREDIENTS AND APPEARANCE
MACI
autologous cultured chondrocytes implantProduct Information Product Type CELLULAR THERAPY Item Code (Source) NDC:69866-1030 Route of Administration INTRA-ARTICULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AUTOLOGOUS CULTURED CHONDROCYTES (UNII: D5P3K3V822) (AUTOLOGOUS CULTURED CHONDROCYTES - UNII:D5P3K3V822) AUTOLOGOUS CULTURED CHONDROCYTES 15000000 PORK COLLAGEN (UNII: I8442U2G7J) (PORK COLLAGEN - UNII:I8442U2G7J) PORK COLLAGEN 15 cm2 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69866-1030-5 1 in 1 BOX 1 NDC:69866-1030-4 1 in 1 BAG 1 NDC:69866-1030-3 1 in 1 BOTTLE, PLASTIC; Type 5: Device Coated or Otherwise Combined with Biologic 2 NDC:69866-1030-8 2 in 1 BOX 2 NDC:69866-1030-7 1 in 1 BAG 2 NDC:69866-1030-6 1 in 1 BOTTLE, PLASTIC; Type 5: Device Coated or Otherwise Combined with Biologic Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125603 06/09/2017 Labeler - Vericel Corporation (079745570)