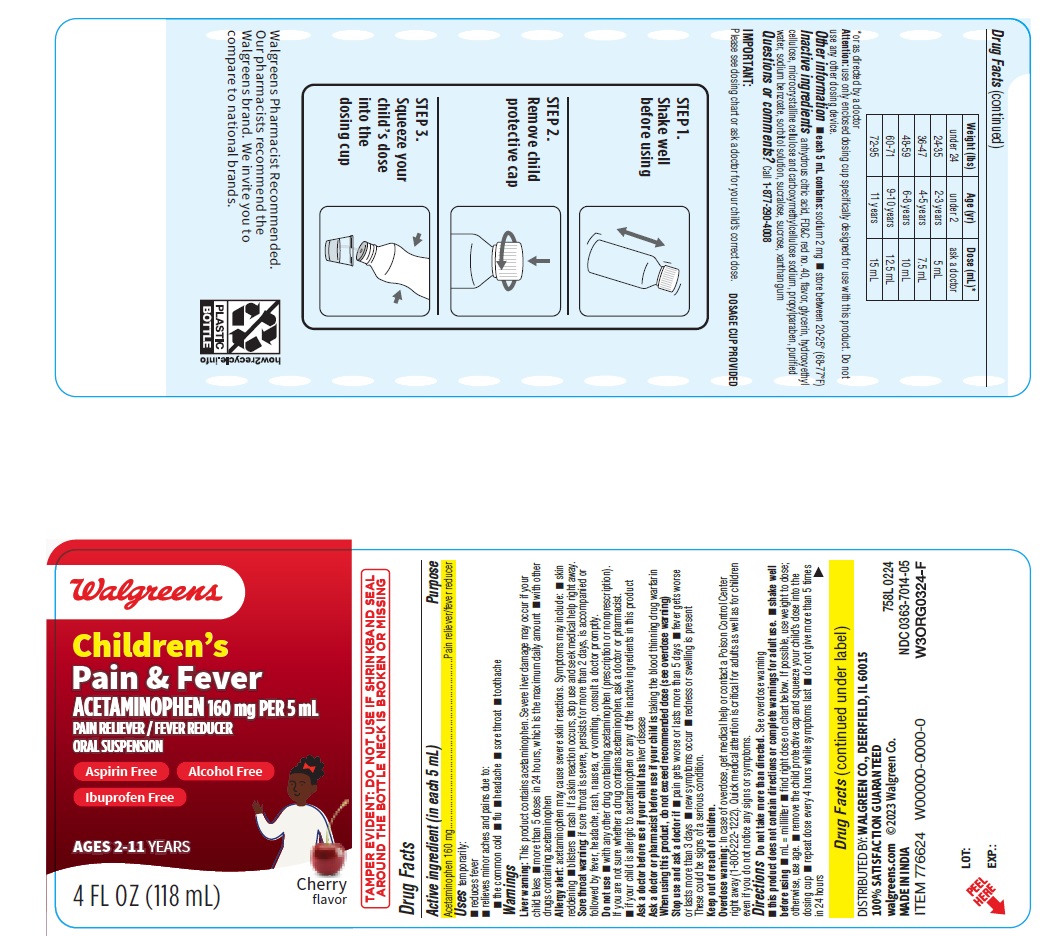
Label: CHILDRENS PAIN AND FEVER- acetaminophen suspension
- NDC Code(s): 0363-7014-05
- Packager: WALGREENS
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
- DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
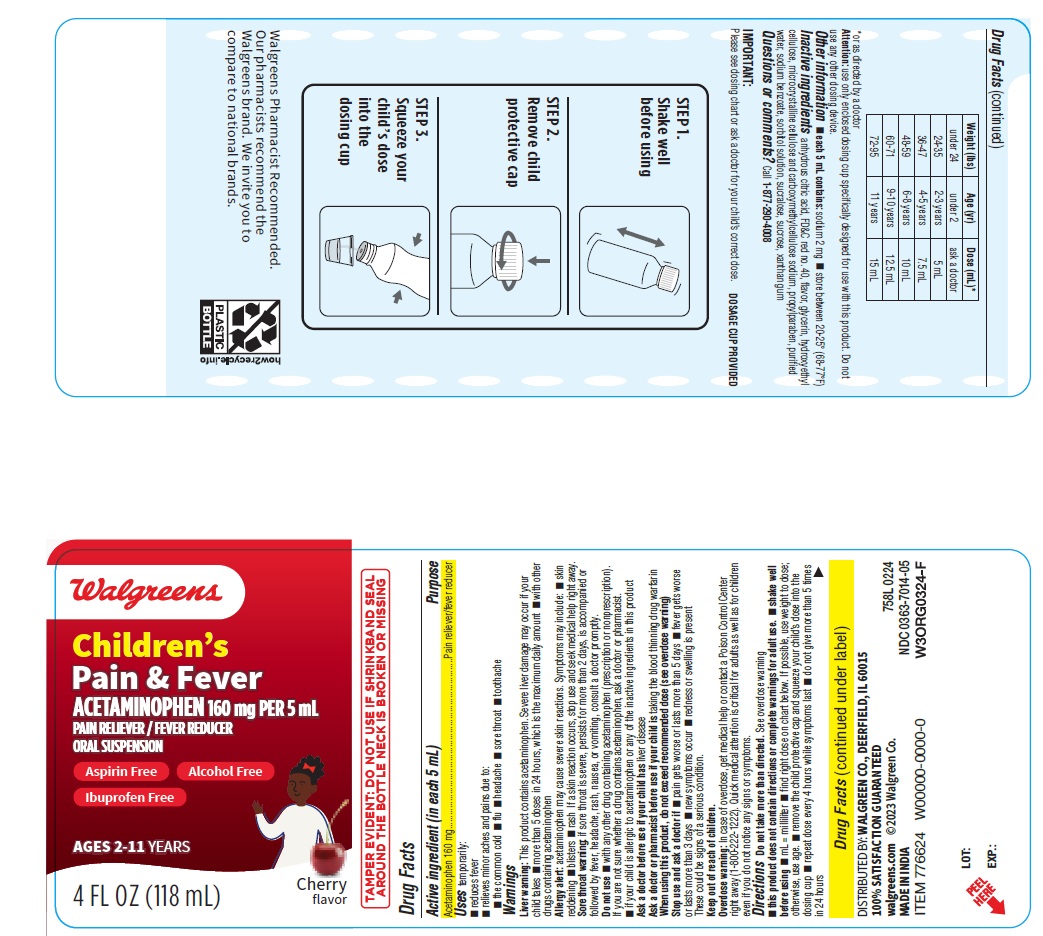
DOSAGE & ADMINISTRATION
Directions
Do not take more than directed.
See overdose warning
- this product does not contain directions or complete warnings for adult use.
- shake well before using
- mL = milliliter
- find right dose on chart below. If possible, use weight to dose; otherwise, use age.
- remove the child protective cap and squeeze your child’s dose into the dosing cup
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
Weight (lbs) Age (yr) Dose (mL)* under 24 under 2 ask a doctor 24-35 2-3 years 5 mL 36-47 4-5 years 7.5 mL 48-59 6-8 years 10 mL 60-71 9-10 years 12.5 mL 72-95 11 years 15 mL *or as directed by a doctor
Attention: use only enclosed dosing cup specifically designed for use with this product. Do not use any other dosing device.
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHILDRENS PAIN AND FEVER
acetaminophen suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-7014 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg in 5 mL Inactive Ingredients Ingredient Name Strength FD&C RED NO. 40 (UNII: WZB9127XOA) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) GLYCERIN (UNII: PDC6A3C0OX) SORBITOL SOLUTION (UNII: 8KW3E207O2) SUCROSE (UNII: C151H8M554) Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-7014-05 1 in 1 CARTON 04/10/2024 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 04/10/2024 Labeler - WALGREENS (008965063) Registrant - TIME CAP LABORATORIES, INC. (037052099) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LIMITED 677604129 manufacture(0363-7014)