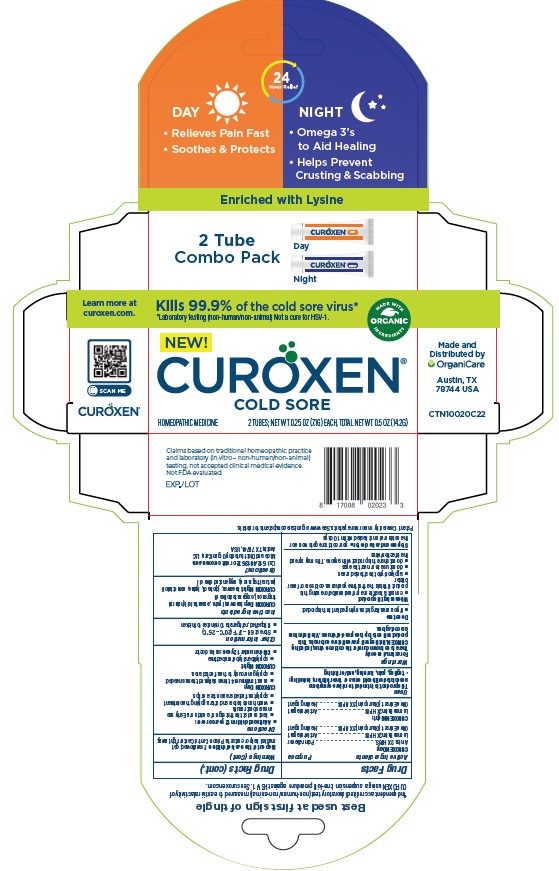
Label: CUROXEN COLD SORE DAY ointment
- NDC Code(s): 71042-014-14
- Packager: OrganiCare Nature's Science, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active Ingredients
Active ingredients Purpose
CUROXEN Day:
Arnica 3X HPUS..............................................Pain reliever
Lemon Balm 2X HPUS ....................................Antiviral agent
Olive Extract (Oleuropein) 3X HPUS ...............Healing agent
CUROXEN Night:
Lemon Balm 2X HPUS ....................................Antiviral agent
Olive Extract (Oleuropein) 3X HPUS ...............Healing agent - Ask a doctor
- Do Not Use
- Keep out of the reach of children.
- Uses
- Questions
- Stop use and ask a doctor
-
Directions
Directions
Adults and children 12 years or over:
best used at the first sign of a cold sore. Early use
ensures best results
wash hands before and after applying the ointment
apply to affected area on face or lips
CUROXEN Day:
use at minimum 4 times daily and then as needed
apply generously to the affected area
CUROXEN Night:
apply liberally before bedtime
Children under 12 years: ask a doctor - When Using this Product
- Inactive Ingredients
- Other Information
- Warnings
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CUROXEN COLD SORE DAY
curoxen cold sore day ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71042-014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEMON BALM OIL (UNII: MJ76269K9S) (LEMON BALM OIL - UNII:MJ76269K9S) LEMON BALM OIL 1 g in 100 g ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 0.1 g in 100 g Inactive Ingredients Ingredient Name Strength OLIVE OIL (UNII: 6UYK2W1W1E) 94.8 g in 100 g VETIVER OIL (UNII: 9M9P32M01L) 2 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71042-014-14 1 in 1 CARTON 12/02/2021 1 14.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/02/2021 Labeler - OrganiCare Nature's Science, LLC (044204745) Establishment Name Address ID/FEI Business Operations OrganiCare Nature's Science, LLC 044204745 manufacture(71042-014)