Label: TERBUTALINE SULFATE tablet
- NDC Code(s): 71205-938-00, 71205-938-30, 71205-938-55, 71205-938-60, view more
- Packager: Proficient Rx LP
- This is a repackaged label.
- Source NDC Code(s): 24979-132, 24979-133
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only
-
BOXED WARNING
(What is this?)
WARNING: TOCOLYSIS
Oral terbutaline sulfate has not been approved and should not be used for acute or maintenance tocolysis. In particular, terbutaline sulfate should not be used for maintenance tocolysis in the outpatient or home setting. Serious adverse reactions, including death, have been reported after administration of terbutaline sulfate to pregnant women. In the mother, these adverse reactions include increased heart rate, transient hyperglycemia, hypokalemia, cardiac arrhythmias, pulmonary edema and myocardial ischemia. Increased fetal heart rate and neonatal hypoglycemia may occur as a result of maternal administration. [see Contraindications, Tocolysis.]
Close -
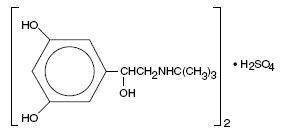
DESCRIPTIONTerbutaline sulfate USP is a beta-adrenergic agonist bronchodilator available as tablets of 2.5 mg (2.05 mg of the free base) and 5 mg (4.1 mg of the free base) for oral administration ...
-
CLINICAL PHARMACOLOGYIn vitro and in vivo pharmacologic studies have demonstrated that terbutaline exerts a preferential effect on beta2-adrenergic receptors. While it is recognized that beta2-adrenergic receptors ...
-
INDICATIONS AND USAGETerbutaline sulfate is indicated for the prevention and reversal of bronchospasm in patients 12 years of age and older with asthma and reversible bronchospasm associated with bronchitis and ...
-
CONTRAINDICATIONS1. Tocolysis - Oral terbutaline sulfate has not been approved and should not be used for acute or maintenance tocolysis. [see Boxed Warning: Tocolysis.] 2. Hypersensitivity - Terbutaline sulfate ...
-
WARNINGSDeterioration of Asthma - Asthma may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient needs more doses of terbutaline sulfate than usual, this ...
-
PRECAUTIONSGeneral - Terbutaline, as with all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, including ischemic heart disease, hypertension, and cardiac ...
-
ADVERSE REACTIONSAdverse reactions observed with terbutaline sulfate are similar to those commonly seen with other sympathomimetic amines. All of these reactions are generally transient in nature and usually do ...
-
DOSAGE AND ADMINISTRATIONAdults - The usual oral dose of terbutaline sulfate for adults is 5 mg administered at approximately six-hour intervals, three times daily, during the hours the patient is usually awake. If side ...
-
OVERDOSAGEThe median subcutaneous lethal dose of terbutaline sulfate in mature rats is approximately 165 mg/kg (approximately 90 times the maximum recommended daily oral dose for adults on a mg/m2 basis) ...
-
HOW SUPPLIEDTerbutaline sulfate tablets, USP are packaged in bottles of 100 and 1000 tablets. Descriptions of the 2.5 and 5 mg tablets follow: Tablets 2.5 mg - White to off-white, oval tablets, scored on one ...
-
PRINCIPAL DISPLAY PANEL — 2.5 mgNDC 71205-938-30 - TERBUTALINE SULFATE TABLETS, USP - 2.5 mg - Rx Only - 30 TABLETS
-
PRINCIPAL DISPLAY PANEL — 5 mgNDC 71205-939-30 - TERBUTALINE SULFATE TABLETS, USP - 5 mg - Rx Only - 30 TABLETS
-
INGREDIENTS AND APPEARANCEProduct Information