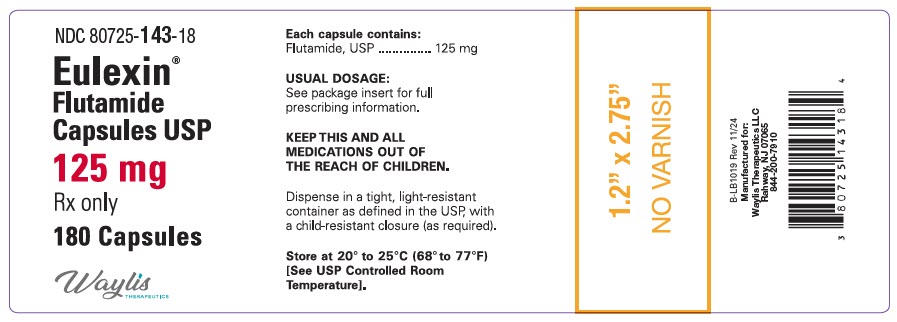
Label: EULEXIN- flutamide capsule
- NDC Code(s): 80725-143-18
- Packager: Waylis Therapeutics LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 4, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only
-
BOXED WARNING
(What is this?)Hepatic Injury - There have been postmarketing reports of hospitalization and rarely death due to liver failure in patients taking Eulexin® . Evidence of hepatic injury included elevated serum ...
WARNINGS
CloseHepatic Injury
There have been postmarketing reports of hospitalization and rarely death due to liver failure in patients taking Eulexin® . Evidence of hepatic injury included elevated serum transaminase levels, jaundice, hepatic encephalopathy and death related to acute hepatic failure. The hepatic injury was reversible after discontinuation of therapy in some patients. Approximately half of the reported cases occurred within the initial 3 months of treatment with Eulexin®.
Serum transaminase levels should be measured prior to starting treatment with Eulexin®. Eulexin® is not recommended in patients whose ALT values exceed twice the upper limit of normal. Serum transaminase levels should then be measured monthly for the first 4 months of therapy, and periodically thereafter. Liver function tests also should be obtained at the first signs and symptoms suggestive of liver dysfunction, e.g., nausea, vomiting, abdominal pain, fatigue, anorexia, "flu-like" symptoms, hyperbilirubinuria, jaundice or right upper quadrant tenderness. If at any time, a patient has jaundice, or their ALT rises above 2 times the upper limit of normal, Eulexin® should be immediately discontinued with close follow-up of liver function tests until resolution.
-
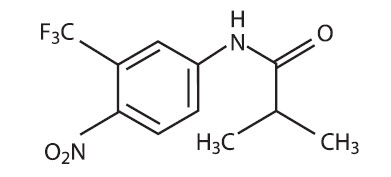
DESCRIPTIONEulexin® capsules contain flutamide, an acetanilid, nonsteroidal, orally active antiandrogen having the chemical name, α,α,α-trifluoro-2-methyl-4'-nitro-m-propionotoluidide. Each capsule contains ...
-
CLINICAL PHARMACOLOGYGeneral - In animal studies, flutamide demonstrates potent antiandrogenic effects. It exerts its antiandrogenic action by inhibiting androgen uptake and/or by inhibiting nuclear binding of ...
-
CLINICAL STUDIESEulexin® has been demonstrated to interfere with testosterone at the cellular level. This can complement medical castration achieved with LHRH-agonists which suppresses testicular androgen ...
-
INDICATIONS AND USAGEEulexin® capsules are indicated for use in combination with LHRH-agonists for the management of locally confined Stage B2-C and Stage D2 metastatic carcinoma of the prostate. Stage B2-C ...
-
CONTRAINDICATIONSEulexin® capsules are contraindicated in patients who are hypersensitive to Eulexin® or any component of this preparation. Eulexin® capsules are contraindicated in patients with severe hepatic ...
-
WARNINGSHepatic Injury - SEE BOXED WARNINGS - Use in Women - Eulexin® capsules are for use only in men. This product has no indication for women and should not be used in this population, particularly ...
-
PRECAUTIONSGeneral - In clinical trials, gynecomastia occurred in 9% of patients receiving Eulexin® together with medical castration. Information for Patients - Patients should be informed that Eulexin ...
-
ADVERSE REACTIONSStage B2-C Prostatic Carcinoma - Treatment with Eulexin® capsules and the goserelin acetate implant did not add substantially to the toxicity of radiation treatment alone. The following adverse ...
-
OVERDOSAGEIn animal studies with Eulexin® alone, signs of overdose included hypoactivity, piloerection, slow respiration, ataxia, and/or lacrimation, anorexia, tranquilization, emesis, and ...
-
DOSAGE AND ADMINISTRATIONThe recommended dosage is 2 capsules 3 times a day at 8 hour intervals for a total daily dose of 750 mg.
-
HOW SUPPLIEDEulexin® capsules USP, 125 mg, are available as opaque, beige/beige capsules, imprinted "w 753" on the cap and body. They are available in bottle of 180 (NDC 80725-143-18). Store at 20° to 25°C ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Waylis Therapeutics LLC - Wixom, MI 48393
-
INFORMATION FOR PATIENTSEULEXIN® (FLUTAMIDE) CAPSULES USP RX ONLYImportant information for patients taking Eulexin® capsules. Read this information carefully each time your prescription is refilled because there may be new information available. This summary ...
-
PRINCIPAL DISPLAY PANEL - 125 mg Capsule Bottle LabelNDC 80725-143-18 - Eulexin® Flutamide - Capsules, USP - 125 mg - Rx only - 180 Capsules - Waylis - THERAPEUTICS
-
INGREDIENTS AND APPEARANCEProduct Information