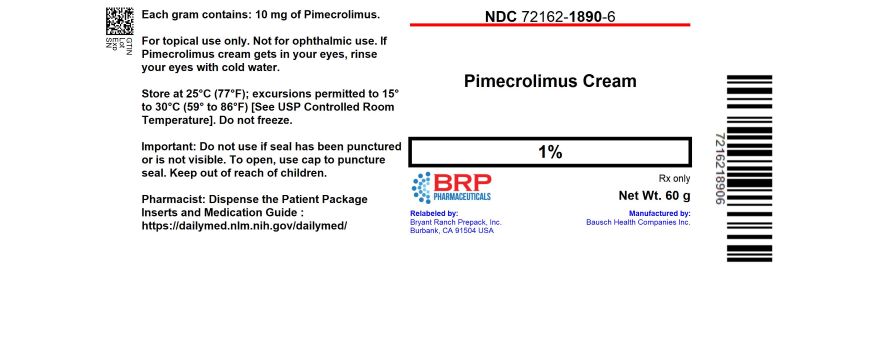
Label: PIMECROLIMUS cream
- NDC Code(s): 72162-1890-6
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 68682-111
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated March 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PIMECROLIMUS safely and effectively. See full prescribing information for PIMECROLIMUS. PIMECROLIMUS cream, for topical use - Initial ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: LONG-TERM SAFETY OF TOPICAL CALCINEURIN INHIBITORS HAS NOT BEEN ESTABLISHED
Although a causal relationship has not been established, rare cases of malignancy (e.g., skin and lymphoma) have been reported in patients treated with topical calcineurin inhibitors, including Pimecrolimus Cream, 1%. [see Warnings and Precautions (5.1)].
Therefore:- •
- Continuous long-term use of topical calcineurin inhibitors, including Pimecrolimus Cream, 1%, in any age group should be avoided and application limited to areas of involvement with atopic dermatitis [see Dosage and Administration (2), Warnings and Precautions (5.1)].
- •
- Pimecrolimus Cream, 1% is not indicated for use in children less than 2 years of age [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)].
-
1 INDICATIONS AND USAGEPimecrolimus Cream, 1% is indicated as second-line therapy for the short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis in non-immunocompromised adults and ...
-
2 DOSAGE AND ADMINISTRATIONApply a thin layer of Pimecrolimus Cream, 1% to the affected skin twice daily. The patient should stop using Pimecrolimus Cream, 1% when signs and symptoms (e.g., itch, rash and redness) resolve ...
-
3 DOSAGE FORMS AND STRENGTHSCream, 1%. Each gram of Pimecrolimus Cream, 1% contains 10 mg of pimecrolimus in a whitish cream base.
-
4 CONTRAINDICATIONSPimecrolimus Cream, 1% is contraindicated in individuals with a history of hypersensitivity to pimecrolimus or any of the components of the cream.
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Immunosuppression - Prolonged systemic use of calcineurin inhibitors for sustained immunosuppression in animal studies and transplant patients following systemic administration has ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSPotential interactions between Pimecrolimus Cream, 1% and other drugs, including immunizations, have not been systematically evaluated. Due to low blood levels of pimecrolimus detected in some ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C - There are no adequate and well-controlled studies with Pimecrolimus Cream, 1% in pregnant women. Therefore, Pimecrolimus Cream, 1% should be used during ...
-
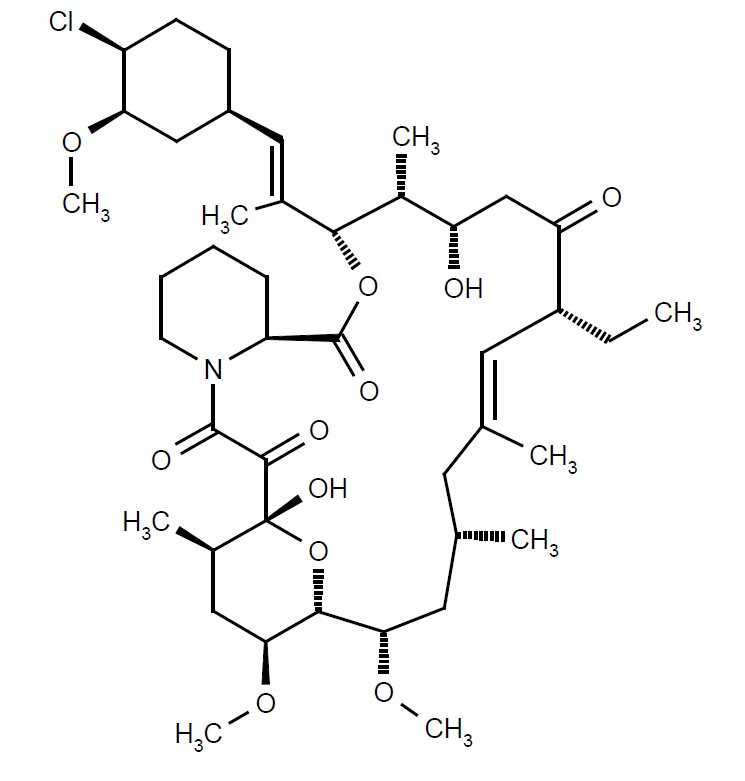
11 DESCRIPTIONPimecrolimus Cream, 1%, for topical use, contains the compound pimecrolimus, the immunosuppressant 33-epi-chloro-derivative of the macrolactam ascomycin. Chemically, pimecrolimus is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of pimecrolimus in atopic dermatitis is not known. While the following have been observed, the clinical significance of these observations in ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 2-year rat dermal carcinogenicity study using Pimecrolimus Cream, 1%, a statistically significant increase in the incidence of ...
-
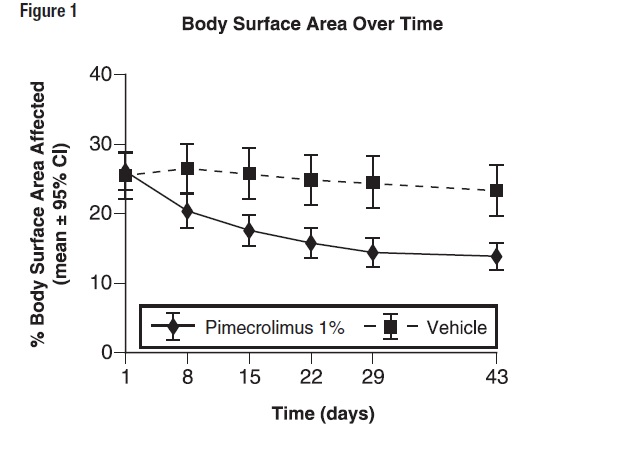
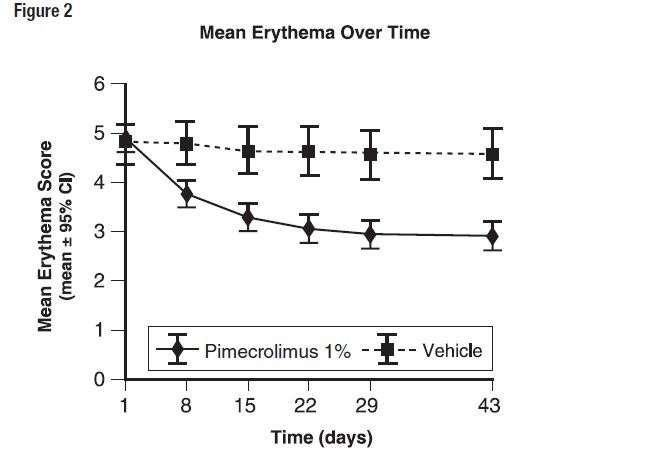
14 CLINICAL STUDIESThree randomized, double-blind, vehicle-controlled, multi-center, Phase 3 trials were conducted in 589 pediatric subjects ages 3 months-17 years old to evaluate Pimecrolimus Cream, 1% for the ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGPimecrolimus Cream, 1% is a whitish cream available in tubes of 60 grams. NDC: 72162-1890-6: 60 g in a TUBE - Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Patients using Pimecrolimus Cream, 1% should receive the following information and instructions: • Pimecrolimus ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Oceanside Pharmaceuticals, a division of - Bausch Health US, LLC - Bridgewater, NJ 08807 USA - Manufactured by: Bausch Health Companies Inc. Laval, Quebec H7L 4A8, Canada - © 2020 ...
-
MEDICATION GUIDEPimecrolimus Cream, 1% Important: Pimecrolimus Cream, 1% is for use on the skin only (topical). Do not get Pimecrolimus Cream, 1% in your eyes, nose, mouth, vagina, or rectum. What is the most ...
-
PRINCIPAL DISPLAY PANELPimecrolimus cream 1% #60
-
INGREDIENTS AND APPEARANCEProduct Information