Label: ALLOPURINOL tablet
- NDC Code(s): 67296-1897-3
- Packager: Redpharm Drug
- This is a repackaged label.
- Source NDC Code(s): 23155-693
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 23, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
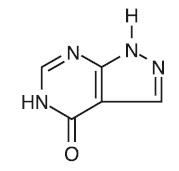
DESCRIPTIONAllopurinol has the following structural formula: Allopurinol is known chemically as 1,5-dihydro-4 - H-pyrazolo [3,4-d]pyrimidin-4-one. It is a xanthine oxidase inhibitor which is ...
-
CLINICAL PHARMACOLOGYAllopurinol acts on purine catabolism, without disrupting the biosynthesis of purines. It reduces the production of uric acid by inhibiting the biochemical reactions immediately preceding its ...
-
INDICATIONS AND USAGETHIS IS NOT AN INNOCUOUS DRUG. IT IS NOT RECOMMENDED FOR THE TREATMENT OF ASYMPTOMATIC HYPERURICEMIA. Allopurinol tablets reduce serum and urinary uric acid concentrations. Its use ...
-
CONTRAINDICATIONSPatients who have developed a severe reaction to allopurinol tablets should not be restarted on the drug.
-
WARNINGSALLOPURINOL TABLETS SHOULD BE DISCONTINUED AT THE FIRST APPEARANCE OF SKIN RASH OR OTHER SIGNS WHICH MAY INDICATE AN ALLERGIC REACTION. In some instances a skin rash may be followed by more ...
-
PRECAUTIONSGeneral - An increase in acute attacks of gout has been reported during the early stages of administration of allopurinol tablets, even when normal or subnormal serum uric acid levels have been ...
-
ADVERSE REACTIONSData upon which the following estimates of incidence of adverse reactions are made are derived from experiences reported in the literature, unpublished clinical trials and voluntary reports ...
-
OVERDOSAGEMassive overdosing or acute poisoning by allopurinol tablets has not been reported. In mice, the 50% lethal dose (LD - 50) is 160 mg/kg given intraperitoneally (IP) with deaths delayed ...
-
DOSAGE AND ADMINISTRATIONThe dosage of allopurinol tablets to accomplish full control of gout and to lower serum uric acid to normal or near-normal levels varies with the severity of the disease. The average is 200 to ...
-
HOW SUPPLIED100-mg (white) scored, flat cylindrical tablets with "I" and "135" on either side of the break line on one side and plain on other side, bottles of 100 (NDC 23155-693-01) and 1000 (NDC ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL100 mg 100 Tablets Bottle - 100 Tablets NDC 23155-693-01 - Allopurinol Tablets USP - Each scored tablet contains - 100 mg - Rx Only - 100 mg 100 Tablets ...
-
INGREDIENTS AND APPEARANCEProduct Information