Label: ELLENCE- epirubicin hydrochloride injection, solution
- NDC Code(s): 0009-5091-01, 0009-5091-25, 0009-5093-01, 0009-5093-10
- Packager: Pharmacia & Upjohn Company LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 24, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ELLENCE safely and effectively. See full prescribing information for ELLENCE. ELLENCE® (epirubicin hydrochloride injection ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: CARDIAC TOXICITY, SECONDARY MALIGNANCIES, EXTRAVASATION AND TISSUE NECROSIS, and SEVERE MYELOSUPPRESSION
- •
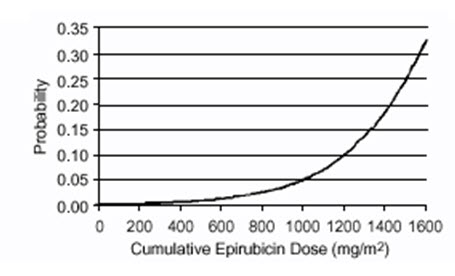
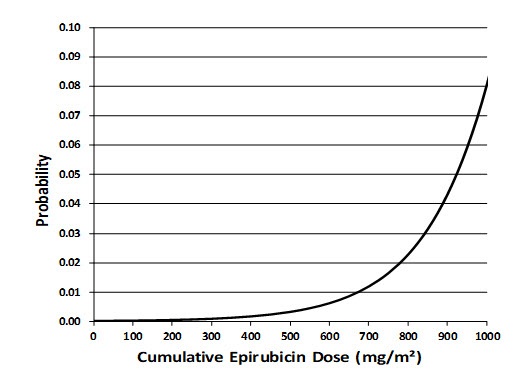
- Cardiac Toxicity: Myocardial damage, including acute left ventricular failure, can occur with ELLENCE. The risk of cardiomyopathy is proportional to the cumulative exposure with incidence rates from 0.9% at a cumulative dose of 550 mg/m2, 1.6% at 700 mg/m2, and 3.3% at 900 mg/m2. The risk of cardiomyopathy is further increased with concomitant cardiotoxic therapy. Assess left ventricular ejection fraction (LVEF) before and regularly during and after treatment with ELLENCE [see Warnings and Precautions (5.1)].
- •
- Secondary Malignancies: Secondary acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) occur at a higher incidence in patients treated with anthracyclines, including ELLENCE [see Warnings and Precautions (5.2)].
- •
- Extravasation and Tissue Necrosis: Extravasation of ELLENCE can result in severe local tissue injury and necrosis requiring wide excision of the affected area and skin grafting. Immediately terminate the drug and apply ice to the affected area [see Warnings and Precautions (5.3)].
- •
- Severe myelosuppression resulting in serious infection, septic shock, requirement for transfusions, hospitalization, and death may occur [see Warnings and Precautions (5.4)].
-
1 INDICATIONS AND USAGEELLENCE is indicated as a component of adjuvant therapy in patients with evidence of axillary node tumor involvement following resection of primary breast cancer [see Clinical Studies ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - When possible, to reduce the risk of developing cardiotoxicity in patients receiving ELLENCE after stopping treatment with other cardiotoxic agents ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: 50 mg/25 mL (2 mg/mL), 200 mg/100 mL (2 mg/mL) clear red solution in a single-dose vial.
-
4 CONTRAINDICATIONSELLENCE is contraindicated in patients with: • Severe myocardial insufficiency [see Warnings and Precautions (5.1)] • Recent myocardial infarction or severe arrhythmias, or previous treatment ...
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiac Toxicity - ELLENCE and other anthracycline drugs can result in either early (or acute) or late (delayed) cardiac toxicity. The principal manifestations of early cardiac toxicity are ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: • Cardiac Toxicity [see Warnings and Precautions (5.1)] • Secondary Malignancies [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Cardiotoxic Agents - Closely monitor cardiac function when ELLENCE is used in combination with other cardiotoxic agents. Patients receiving ELLENCE after stopping treatment with other ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal studies and its mechanism of action, ELLENCE can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology ...
-
10 OVERDOSAGEThere is no known antidote for overdoses of ELLENCE. A 36-year-old man with non-Hodgkin's lymphoma received a daily 95 mg/m2 dose of ELLENCE for 5 consecutive days. Five days later, he developed ...
-
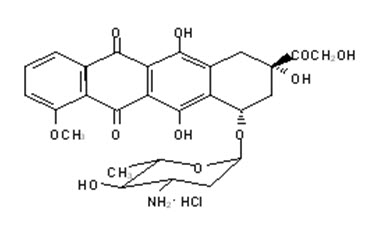
11 DESCRIPTIONELLENCE (epirubicin hydrochloride injection) is an anthracycline topoisomerase inhibitor for intravenous administration. ELLENCE is supplied as a sterile, clear, red solution and is available in ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Epirubicin is an anthracycline cytotoxic agent. Although it is known that anthracyclines can interfere with a number of biochemical and biological functions within ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Conventional long-term animal studies to evaluate the carcinogenic potential of epirubicin have not been conducted, but intravenous ...
-
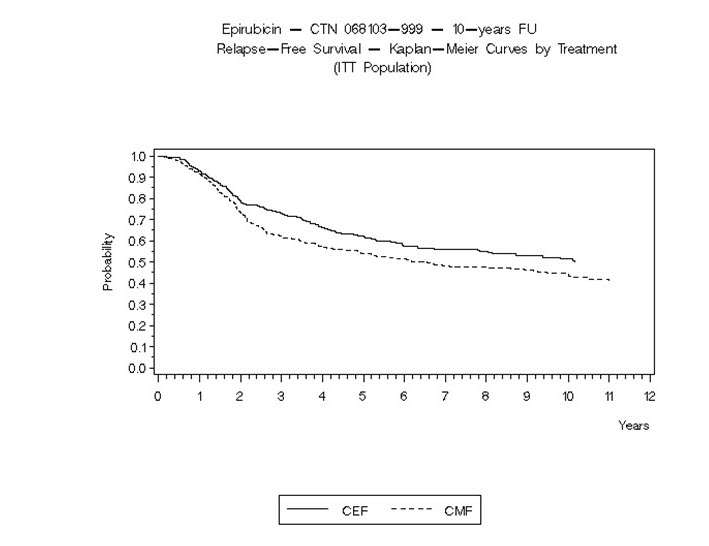
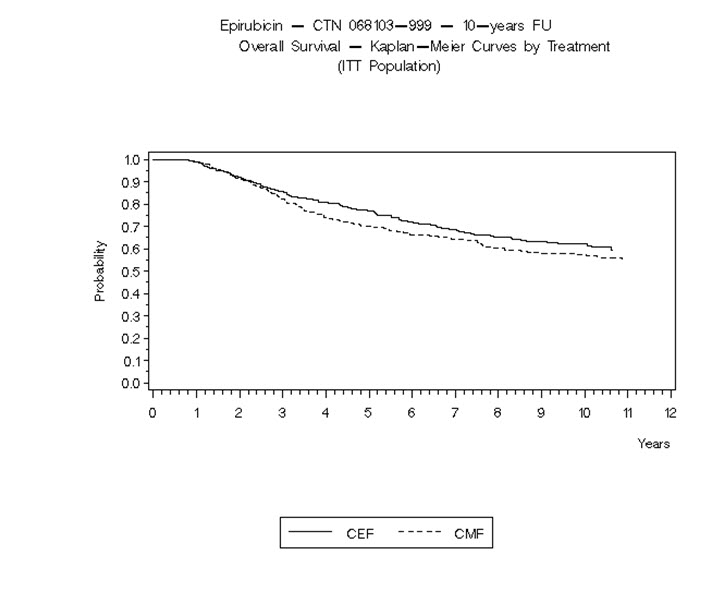
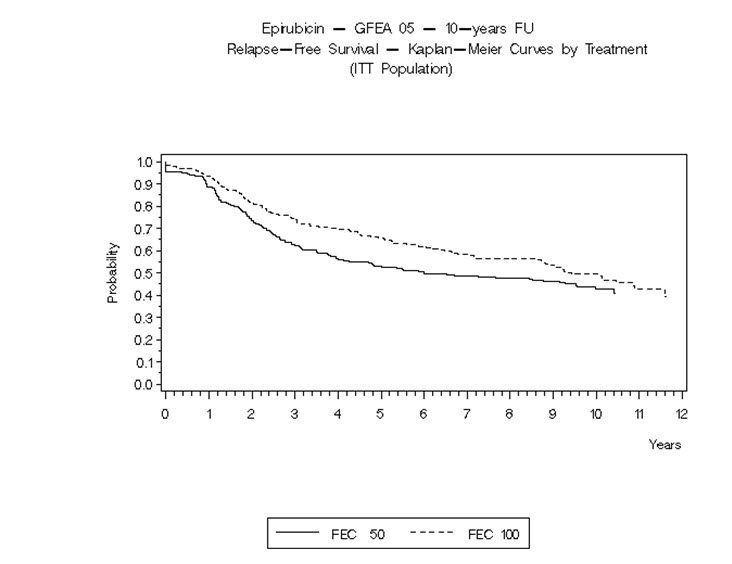
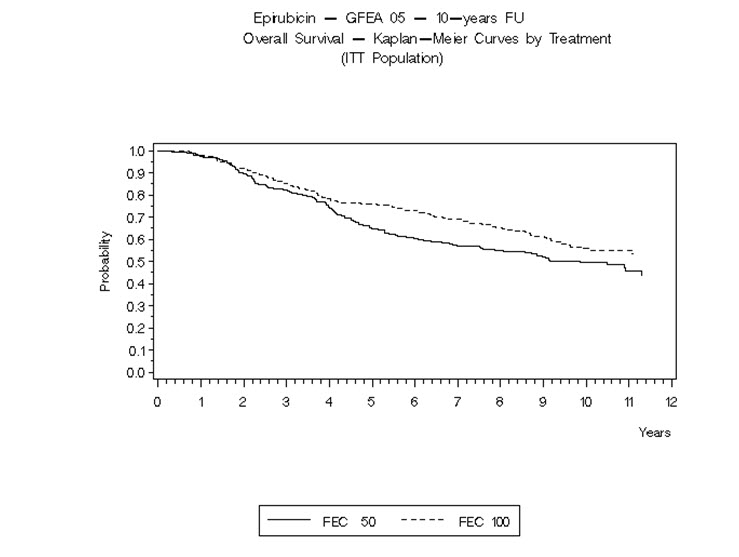
14 CLINICAL STUDIES14.1 Adjuvant Treatment of Breast Cancer - Two randomized, open-label, multicenter studies evaluated the use of ELLENCE 100 to 120 mg/m2 in combination with cyclophosphamide and fluorouracil for ...
-
15 REFERENCES1. "Hazardous Drugs". OSHA.http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLINGELLENCE is available in polypropylene single-dose CYTOSAFE® vials containing 2 mg epirubicin hydrochloride per mL as a sterile, preservative-free, ready-to-use, clear, red solution in the ...
-
17 PATIENT COUNSELING INFORMATIONCardiac Toxicity - Inform patients that there is a risk of irreversible myocardial damage associated with treatment with ELLENCE. Advise patients to immediately contact their healthcare ...
-
SPL UNCLASSIFIED SECTIONThis product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com. For medical information about ELLENCE, please visit www.pfizermedinfo.com or ...
-
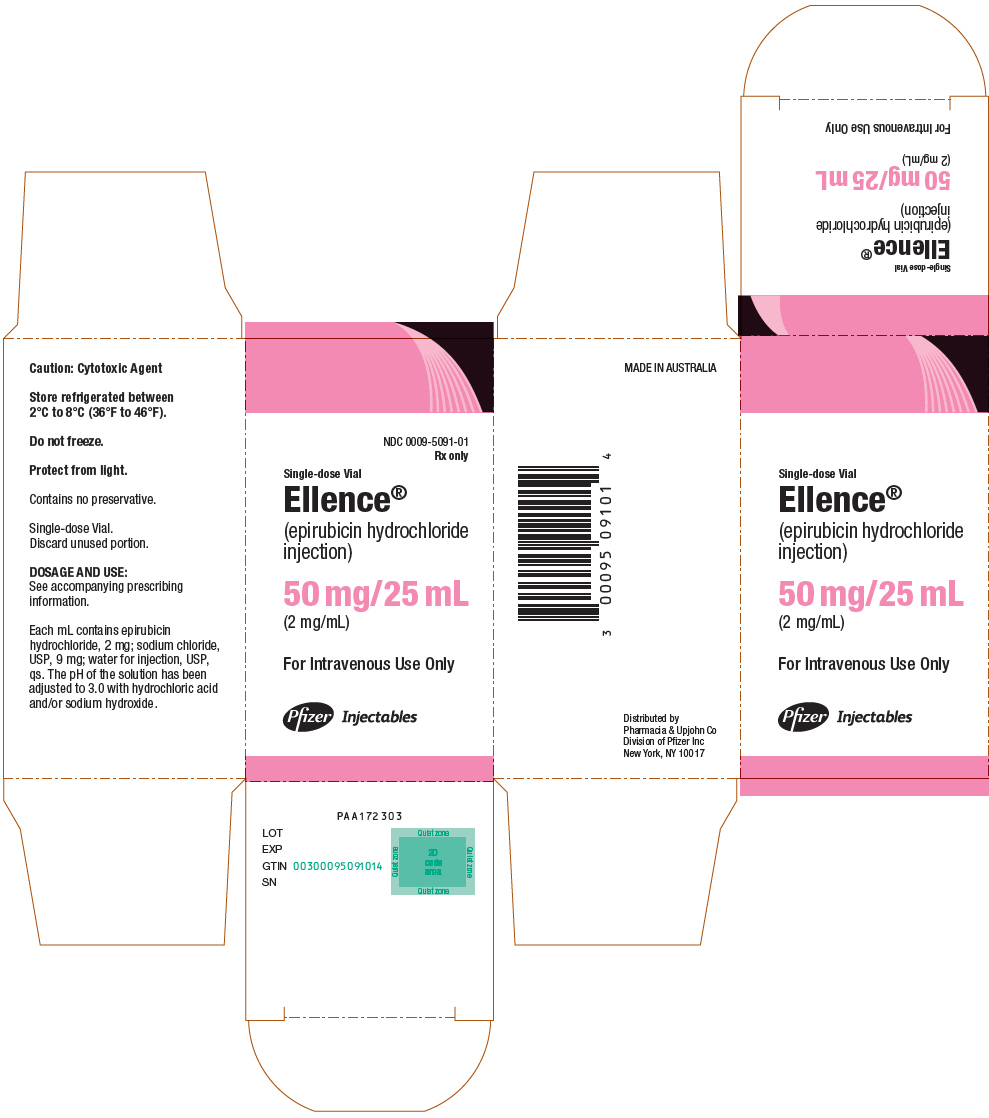
PRINCIPAL DISPLAY PANEL - 50 mg/25 mL Vial LabelNDC 0009-5091-01 - Rx only - Single-dose Vial - Ellence® (epirubicin hydrochloride - injection) 50 mg/25 mL - (2 mg/mL) For Intravenous Use Only
-
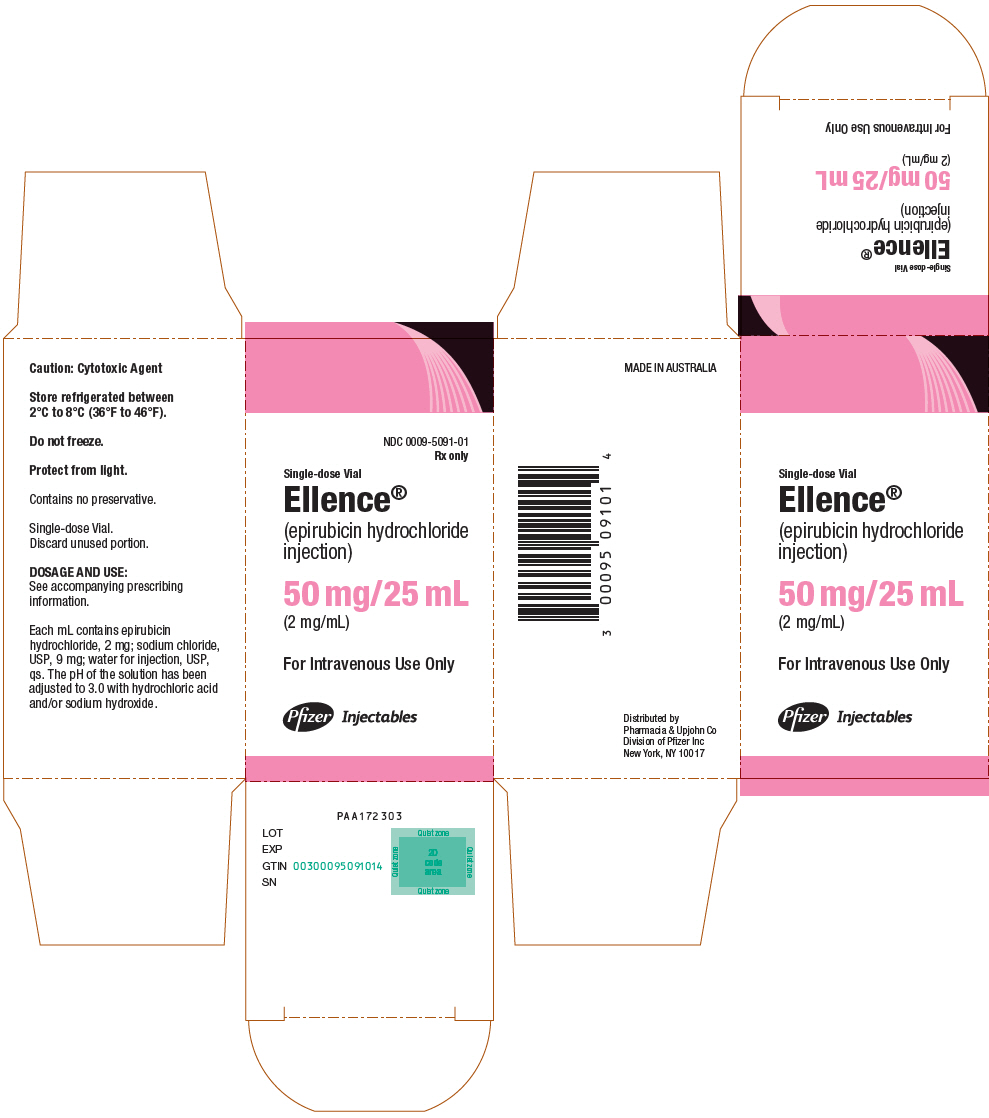
PRINCIPAL DISPLAY PANEL - 50 mg/25 mL Vial CartonNDC 0009-5091-01 - Rx only - Single-dose Vial - Ellence® (epirubicin hydrochloride - injection) 50 mg/25 mL - (2 mg/mL) For Intravenous Use Only - Pfizer Injectables
-
PRINCIPAL DISPLAY PANEL - 200 mg/100 mL Vial LabelNDC 0009-5093-01 - Rx only - Single-dose Vial - Ellence® (epirubicin hydrochloride - injection) 200 mg/100 mL - (2 mg/mL) For Intravenous Use Only - Pfizer Injectables
-
PRINCIPAL DISPLAY PANEL - 200 mg/100 mL Vial CartonNDC 0009-5093-01 - Rx only - Single-dose Vial - Ellence® (epirubicin hydrochloride - injection) 200 mg/100 mL - (2 mg/mL) For Intravenous Use Only - Pfizer Injectables
-
INGREDIENTS AND APPEARANCEProduct Information