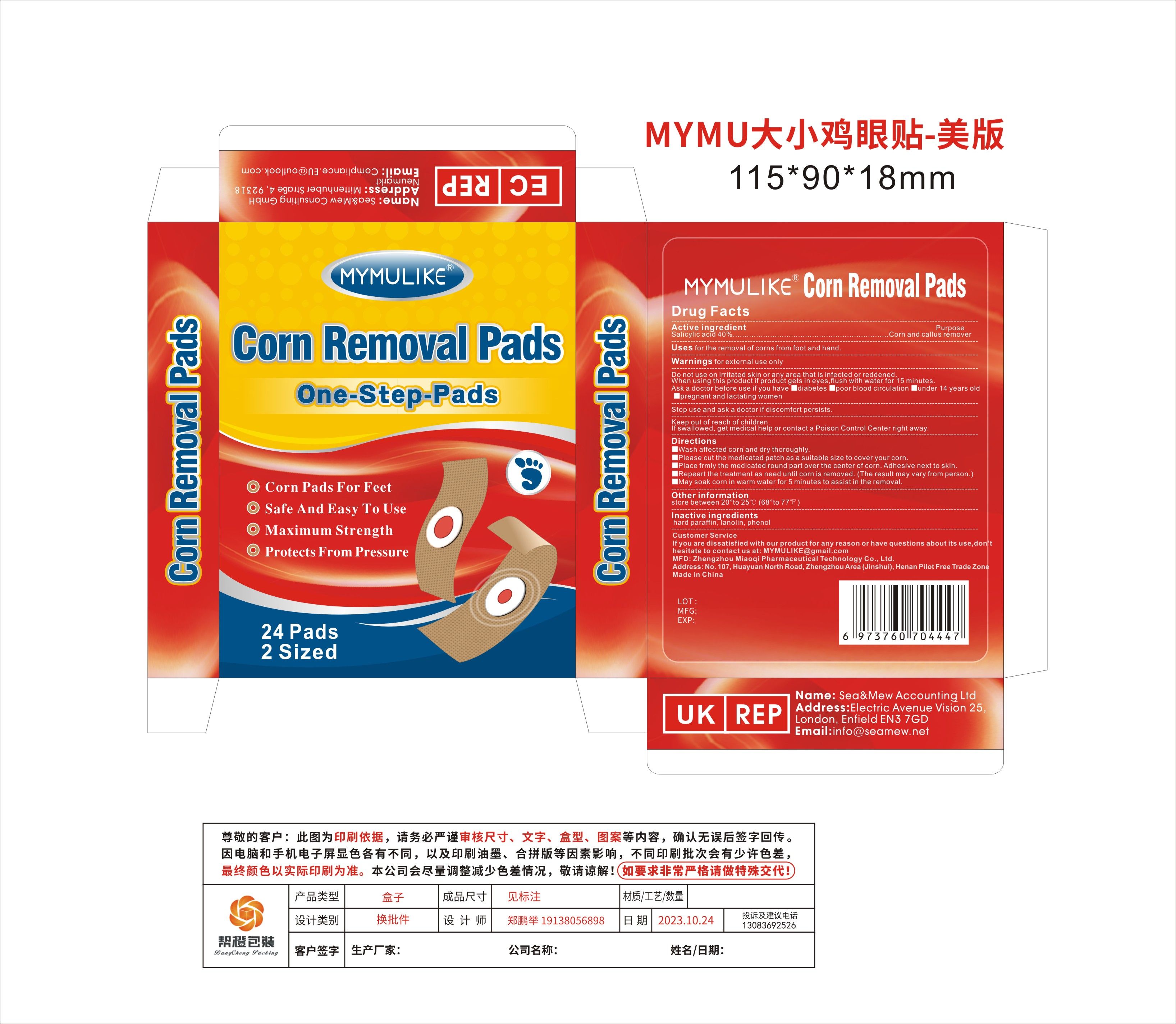
Label: MYMULIKE CORN REMOVAL PADS- corn removal pads patch
- NDC Code(s): 83781-003-01
- Packager: Zhengzhou Miaoqi Pharmaceutical Technology Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 24, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active IngredientSalicylic acid 40%
-
PurposeCorn and callus remover
-
Usefor the removal of corns from foot and hand.
-
Warningsfor external use only
-
Do not useon irritated skin or any area that is infected or reddened.
-
When Usingif product gets in eyes,flush with water for 15 minutes. Ask a doctor before use if you have - ■diabetes - ■poor blood circulation - ■under 14 years old - ■pregnant and lactating women
-
Stop Usediscomfort persists.
-
Ask Doctordiscomfort persists.
-
Keep Out Of Reach Of ChildrenIf swallowed, get medical help or contact a Poison Control Center right away.
-
Directions■Wash affected corn and dry thoroughly. ■Please cut the medicated patch as a suitable size to cover your corn. ■Place frmly the medicated round part over the center of corn. Adhesive next to ...
-
Other informationstore between 20°to 25℃ (68°to 77℉)
-
Inactive ingredientshard paraffin, lanolin, phenol
-
QuestionsMYMULIKE@gmail.com
-
PRINCIPAL DISPLAY PANEL
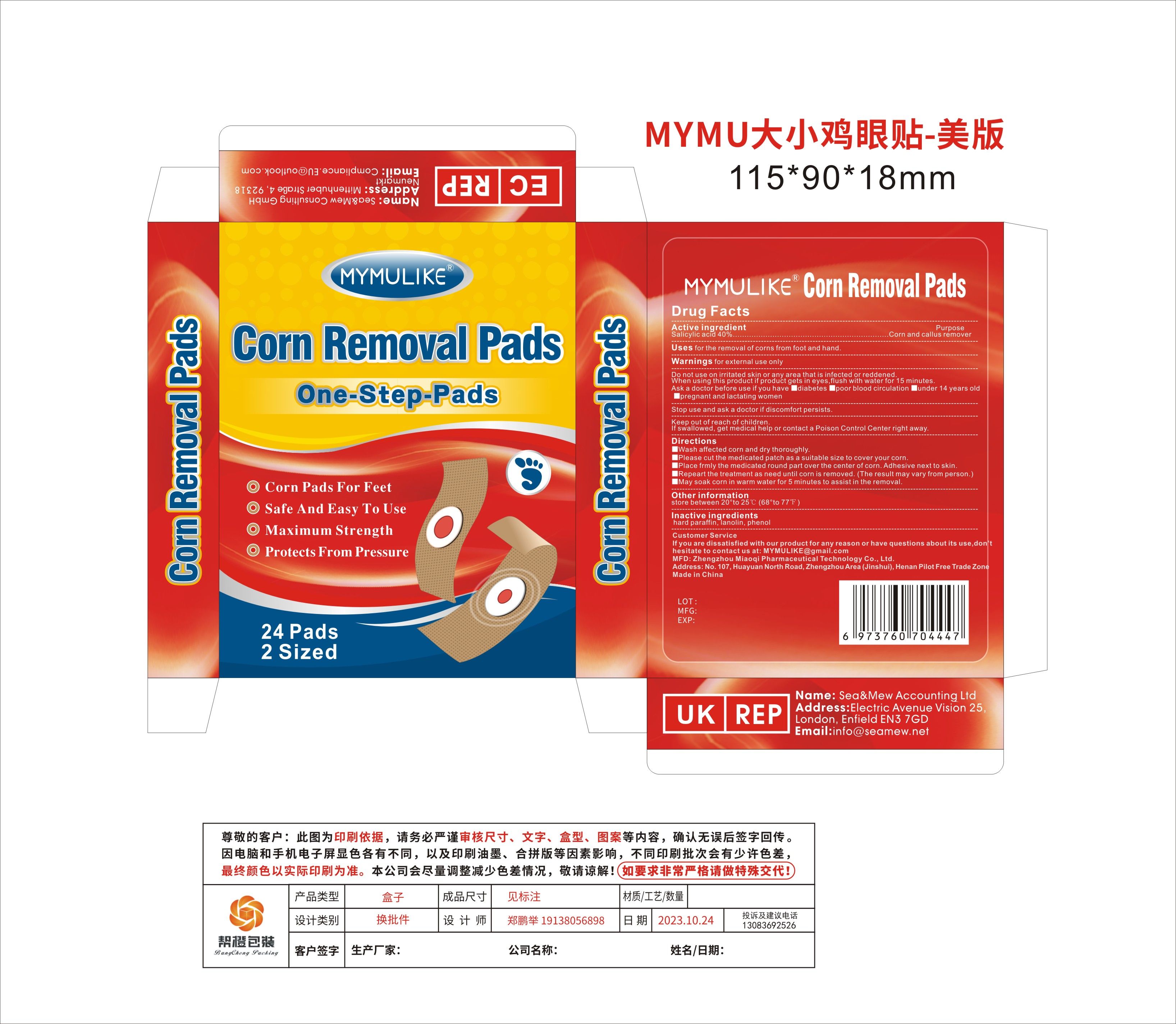
-
INGREDIENTS AND APPEARANCEProduct Information