Label: BYLVAY- odevixibat capsule, coated pellets
BYLVAY- odevixibat capsule
- NDC Code(s): 15054-3301-1, 15054-3302-1, 15054-3303-1, 15054-3304-1
- Packager: Ipsen Biopharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 25, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BYLVAY safely and effectively. See full prescribing information for BYLVAY. BYLVAY (odevixibat) capsules, for oral use - BYLVAY ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Progressive Familial Intrahepatic Cholestasis (PFIC) BYLVAY is indicated for the treatment of pruritus in patients 3 months of age and older with PFIC. Limitations of Use - BYLVAY may ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage for Progressive Familial Intrahepatic Cholestasis (PFIC) in Patients Aged 3 Months and Older - The recommended dosage of BYLVAY is 40 mcg/kg taken orally once daily in the ...
-
3 DOSAGE FORMS AND STRENGTHSOral Pellets: 200 mcg: capsule with ivory opaque cap and white opaque body; imprinted "A200" (black ink). 600 mcg: capsule with ivory opaque cap and body; imprinted "A600" (black ...
-
4 CONTRAINDICATIONSIBAT inhibitors, including BYLVAY, are contraindicated in patients with prior or active hepatic decompensation events (e.g., variceal hemorrhage, ascites, hepatic encephalopathy) [see Warnings ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hepatoxicity - BYLVAY treatment is associated with a potential for drug-induced liver injury (DILI). In the PFIC and ALGS trials, treatment-emergent elevations of liver tests or worsening ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the label: Hepatotoxicity [see Warnings and Precautions (5.1)] Diarrhea [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Bile Acid Binding Resins - Administer bile acid binding resins (e.g., cholestyramine, colesevelam, or colestipol) at least 4 hours before or 4 hours after administration of BYLVAY [see Dosage ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no human data on BYLVAY use in pregnant persons to establish a drug-associated risk of major birth defects, miscarriage, or adverse developmental ...
-
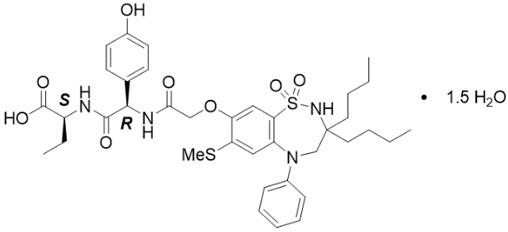
11 DESCRIPTIONThe active ingredient in BYLVAY (odevixibat) capsules and BYLVAY (odevixibat) oral pellets, an ileal bile acid transporter (IBAT) inhibitor, is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Odevixibat is a reversible inhibitor of the ileal bile acid transporter (IBAT). It decreases the reabsorption of bile acids (primarily the salt forms) from the terminal ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In 2-year carcinogenicity studies, odevixibat was not tumorigenic in rats or mice at oral doses up to 100 ...
-
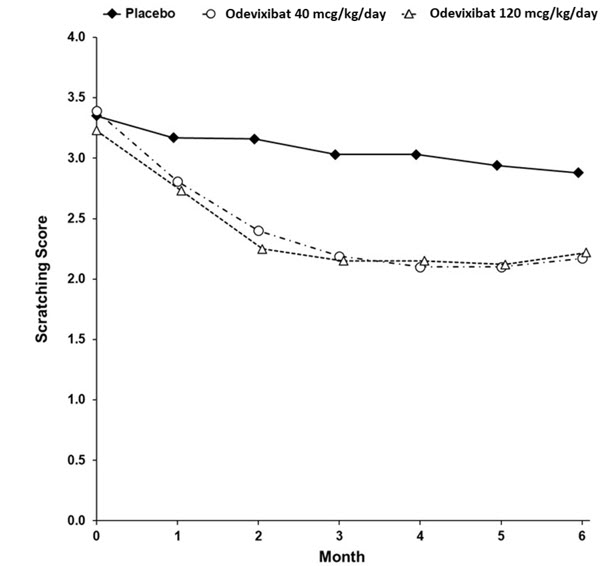
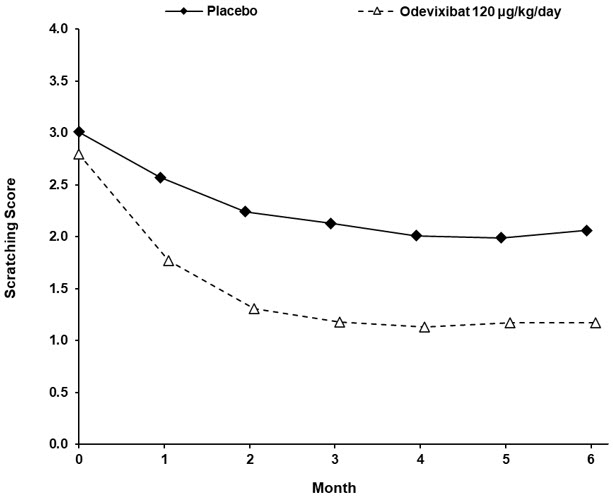
14 CLINICAL STUDIES14.1 PFIC - The efficacy of BYLVAY was evaluated in Trial 1 (NCT03566238), a 24-week, randomized, double-blind, placebo-controlled trial. Trial 1 was conducted in 62 pediatric patients, aged 6 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGOral Pellets - 200 mcg Oral Pellets: supplied as Size 0 capsule with ivory opaque cap and white opaque body; imprinted "A200" (black ink). Supplied in bottles of 30 with child-resistant ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Instructions for Use). Administration Instructions - Advise patients or their caregivers(s) to: Take BYLVAY in the morning with ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Ipsen Biopharmaceuticals, Inc. One Main Street - Cambridge, MA 02142 - © 2025 Ipsen Biopharmaceuticals, Inc. All rights reserved.
-
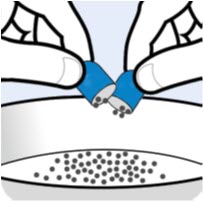
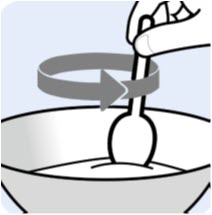
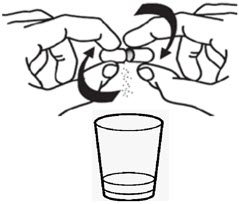
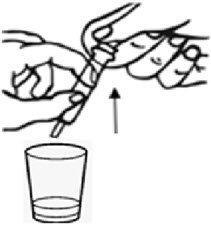
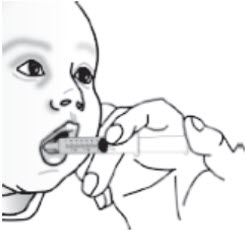
Instructions For UseBYLVAY [bil-vay] (odevixibat)Capsules, for oral useOral PelletsThis Instructions for Use contains information on how to give BYLVAY Capsules and Oral Pellets. This information does not take the place of talking to your healthcare provider about your child's ...
-
PRINCIPAL DISPLAY PANEL - 200 mcg Capsule Bottle CartonNDC 15054-3301-1 - Bylvay® (odevixibat) ORAL PELLETS - 200 mcg - RX ONLY - 30 Capsules
-
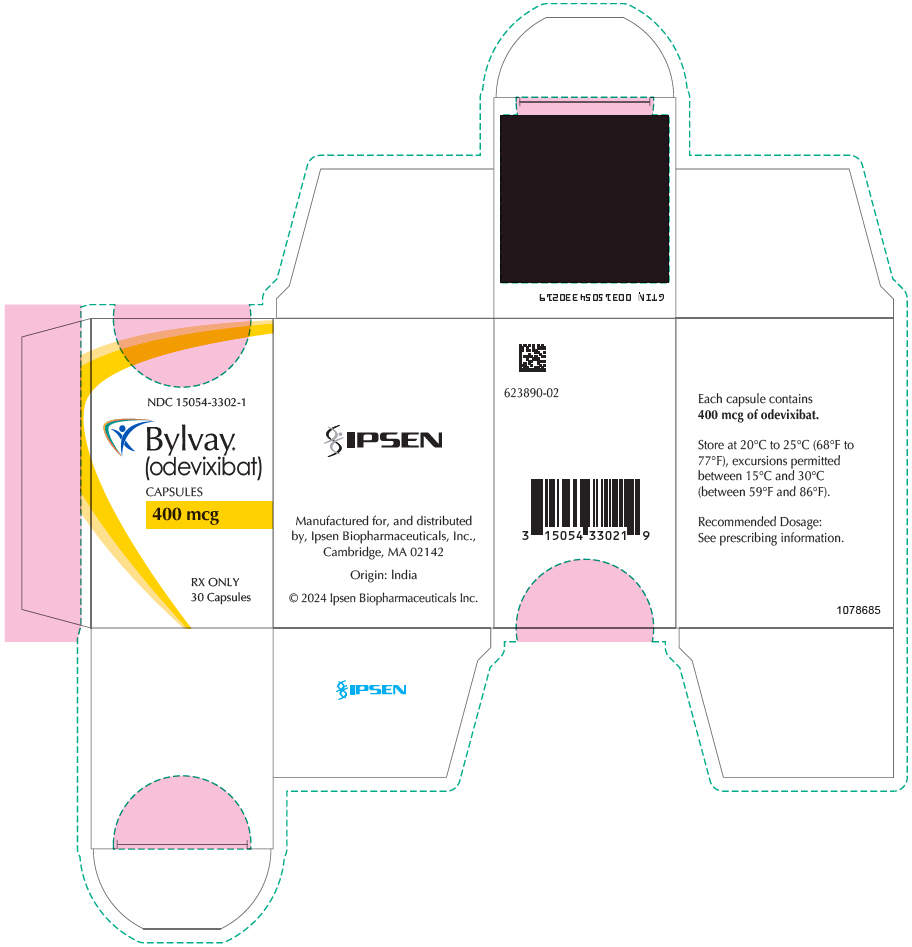
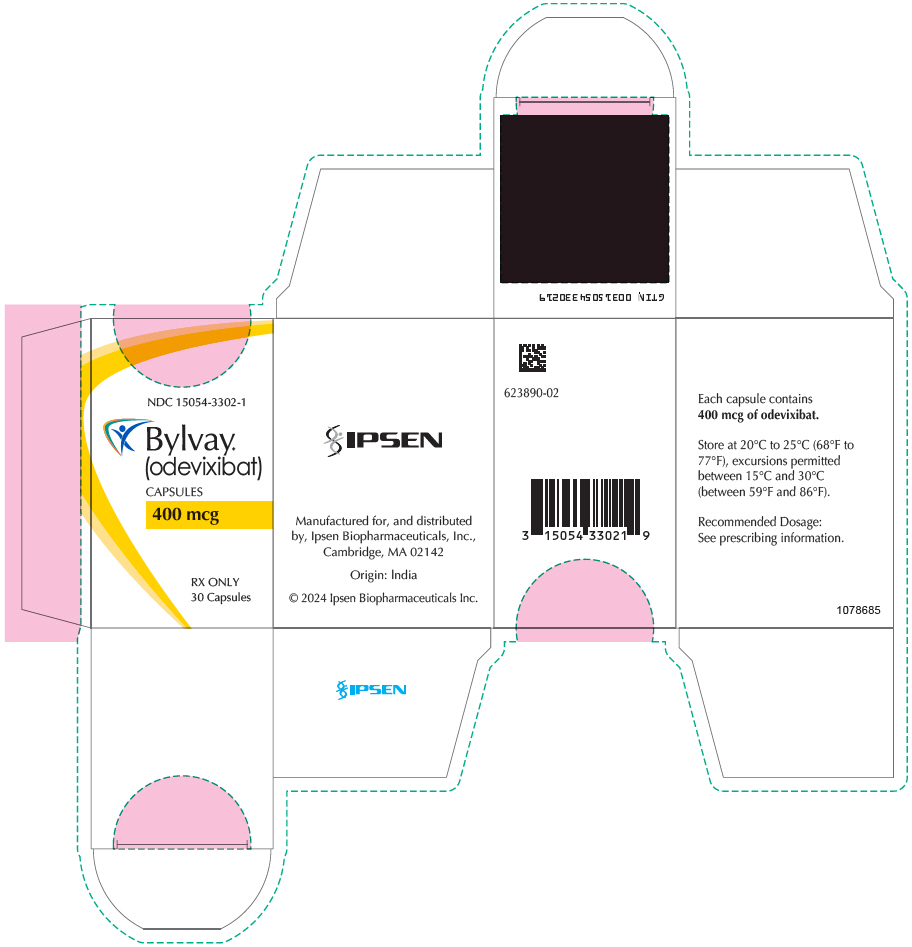
PRINCIPAL DISPLAY PANEL - 400 mcg Capsule Bottle CartonNDC 15054-3302-1 - Bylvay® (odevixibat) CAPSULES - 400 mcg - RX ONLY - 30 Capsules
-
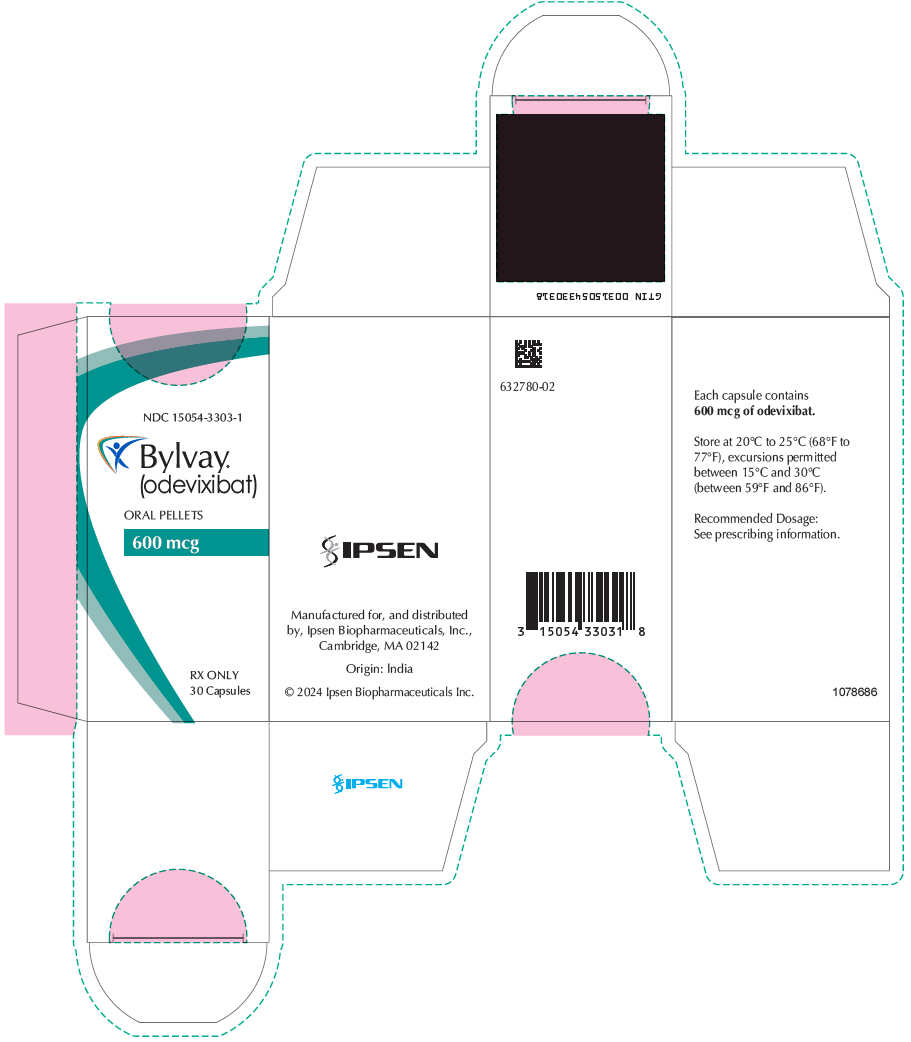
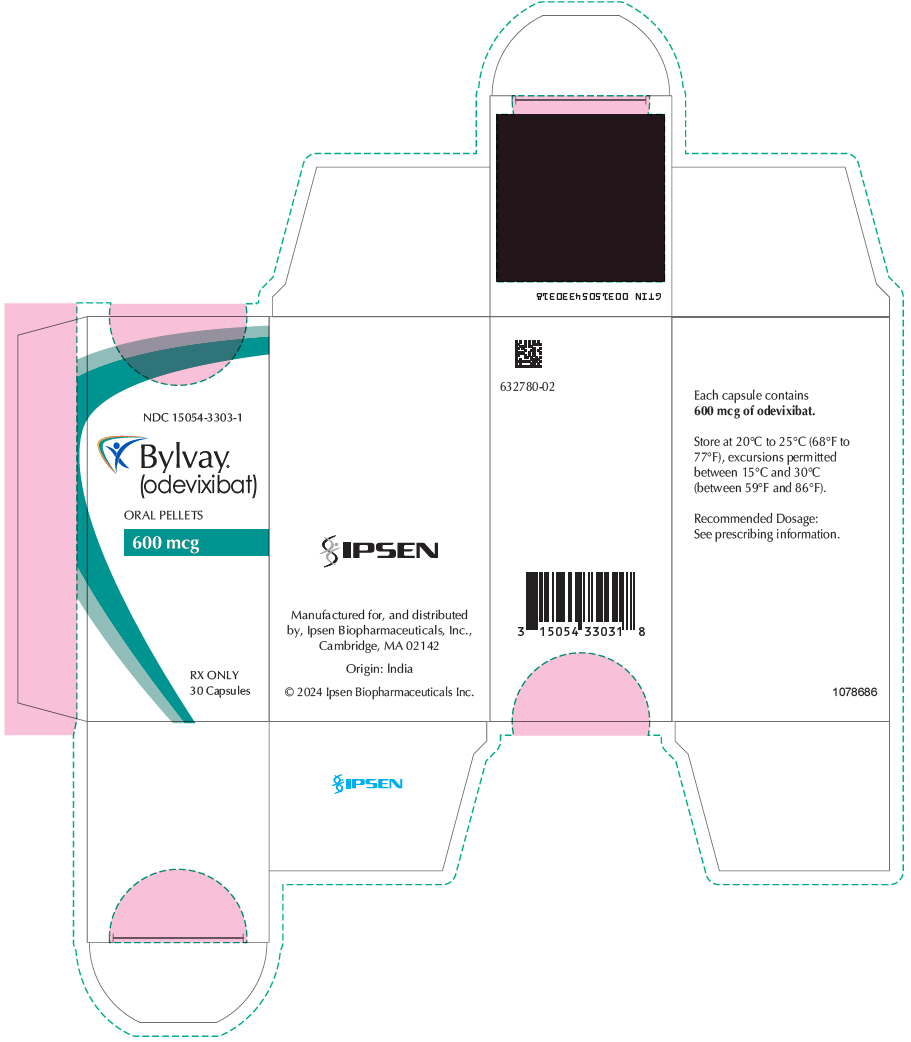
PRINCIPAL DISPLAY PANEL - 600 mcg Capsule Bottle CartonNDC 15054-3303-1 - Bylvay® (odevixibat) ORAL PELLETS - 600 mcg - RX ONLY - 30 Capsules
-
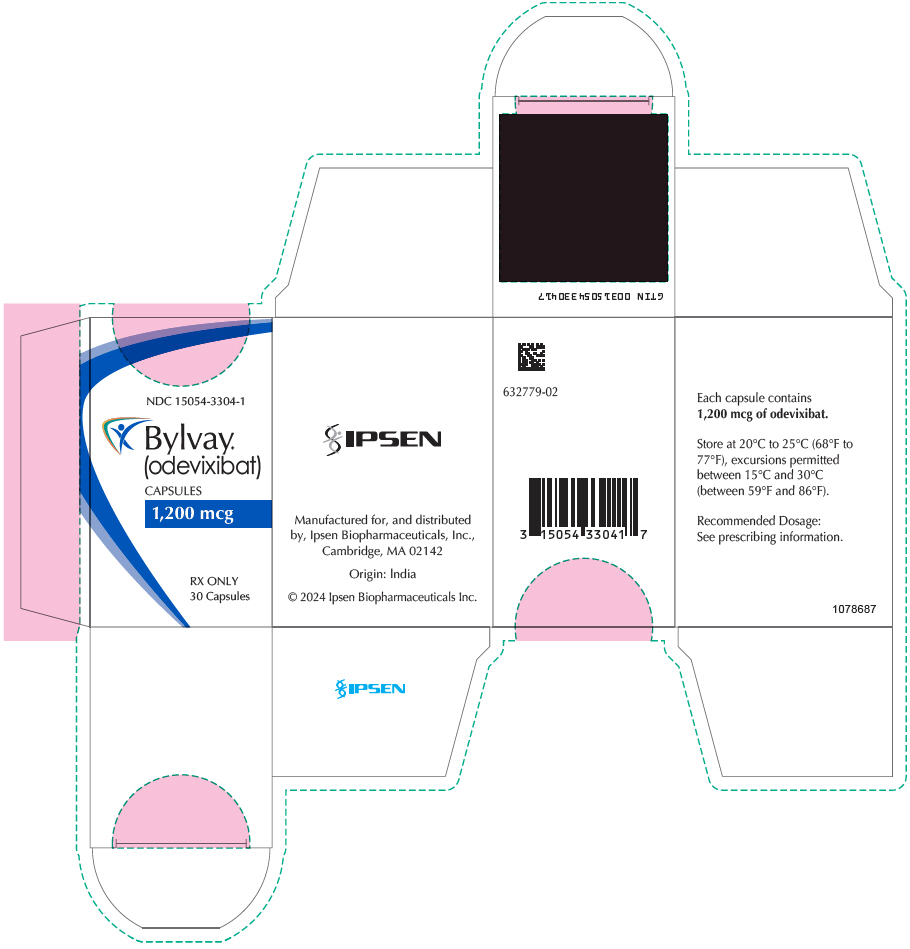
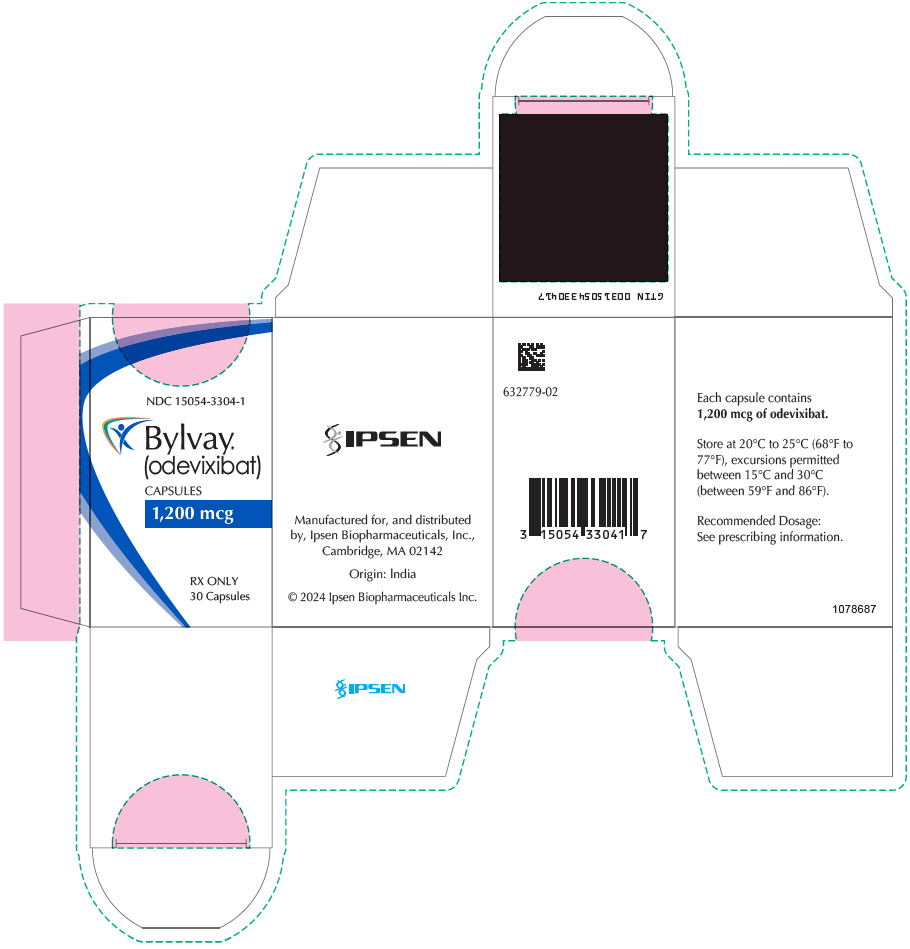
PRINCIPAL DISPLAY PANEL - 1,200 mcg Capsule Bottle CartonNDC 15054-3304-1 - Bylvay® (odevixibat) CAPSULES - 1,200 mcg - RX ONLY - 30 Capsules
-
INGREDIENTS AND APPEARANCEProduct Information