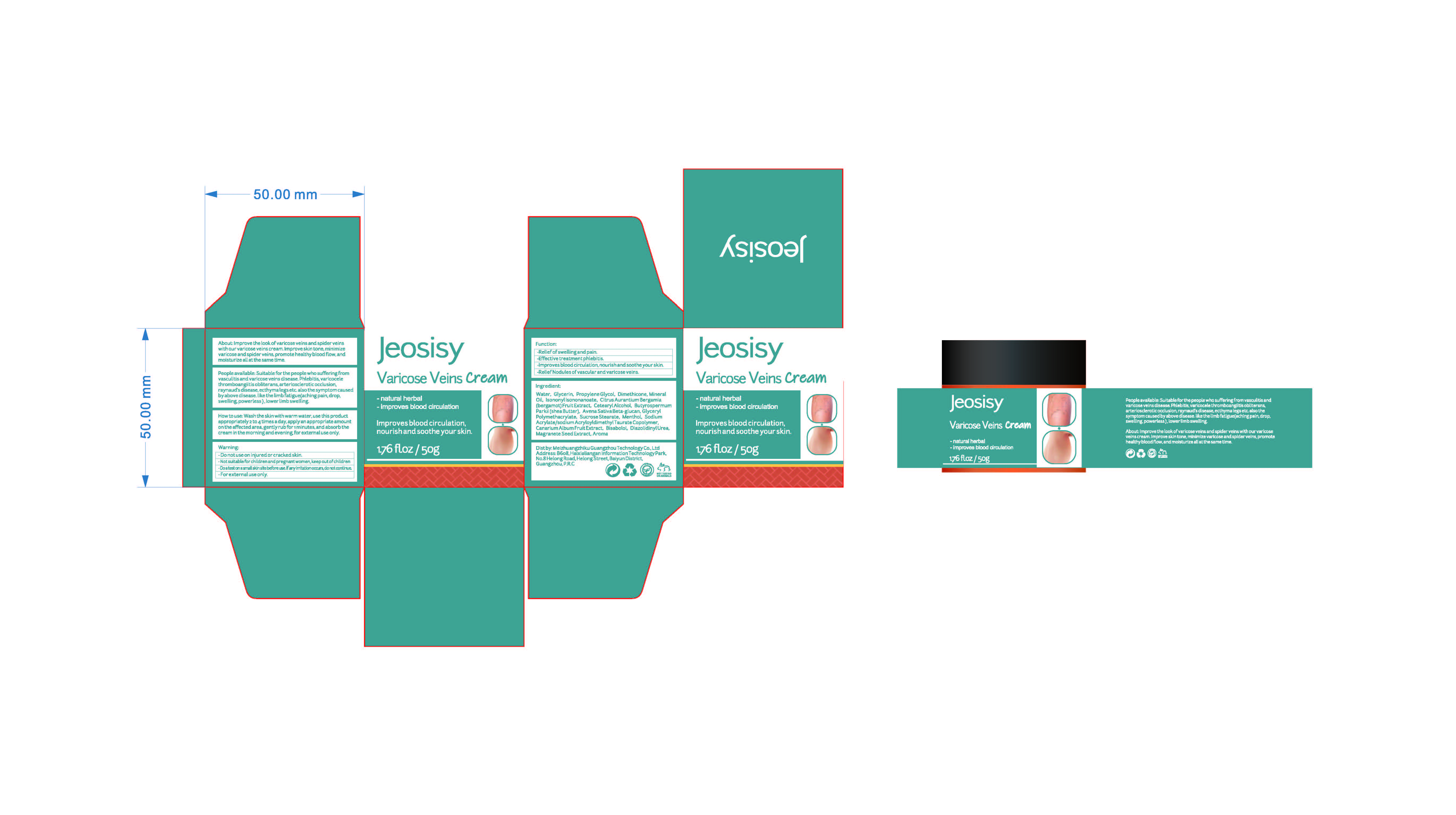
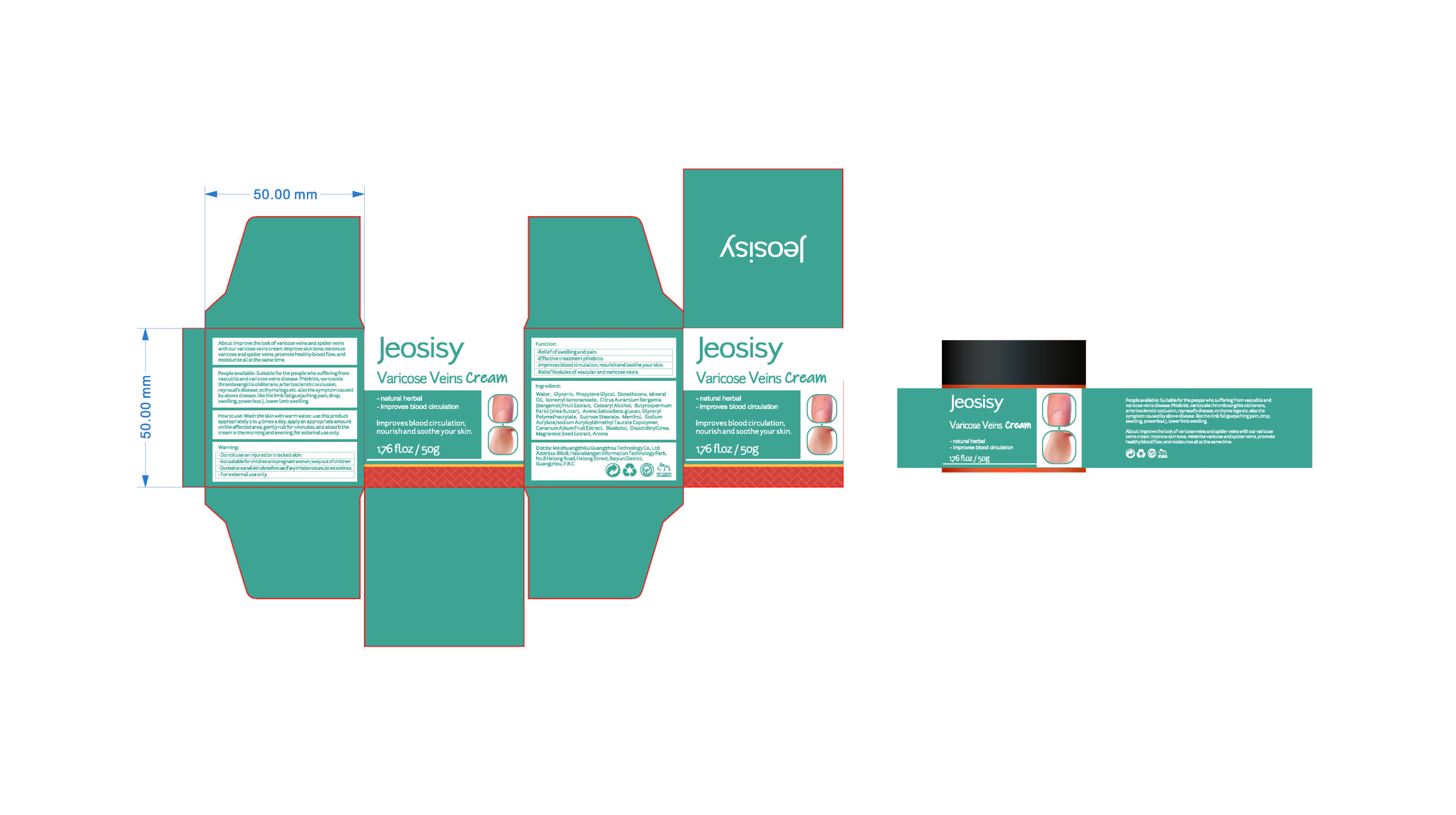
Label: VARICOSE VEINS CREAM- menthol cream
- NDC Code(s): 83782-101-01
- Packager: Meizhuangzhiku Guangzhou Technology Co.,Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
People available: Suitable for the people who sufferingfromvasculitis and varicose veins disease.phlebitis,varicocelethromboangiitis obliterans,arteriosclerotic occlusion.raynaud's disease,ecthyma legs etc.also the symptom causedby above disease.like the limb fatigue(aching pain, drop,swelling,powerless),lower limb swelling
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
ESCIN
CHINESE CINNAMON OIL
ANGELICA ARCHANGELICA ROOT OIL
REHMANNIA GLUTINOSA ROOT EXTRACT
WATER
GLYCERIN
PROPYLENE GLYCOL
DIMETHICONE
MINERAL OIL
ISONONYL ISONONANOATE
CITRUS AURANTIUM BERGAMIA
(BERGAMOT)FRUIT EXTRACT
CETEARYL ALCOHOL
PUNICA GRANATUM SEED EXTRACT
BUTYROSPERMUM
PARKII(SHEA BUTTER)
AVENA SATIVA BETA-GLUCAN
GLYCERYL
POLYMETHACRYLATE
SUCROSE STEARATE
SODIUM
ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER
OLEA EUROPAEA (OLIVE) FRUIT EXTRACT
BISABOLOL
PENTYLENE GLYCOL
DIAZOLIDINYLUREA
AROMA(PRUNUS PERSICA FLOWER) - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VARICOSE VEINS CREAM
menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83782-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AESCULUS CHINENSIS WHOLE (UNII: PGU072XJ6U) (AESCULUS CHINENSIS WHOLE - UNII:PGU072XJ6U) AESCULUS CHINENSIS WHOLE 0.1 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.2 g in 100 g AMMOPIPTANTHUS MONGOLICUS STEM (UNII: 2928MM8X42) (AMMOPIPTANTHUS MONGOLICUS STEM - UNII:2928MM8X42) AMMOPIPTANTHUS MONGOLICUS STEM 0.1 g in 100 g Inactive Ingredients Ingredient Name Strength CHINESE CINNAMON OIL (UNII: A4WO0626T5) MINERAL OIL (UNII: T5L8T28FGP) GLYCERYL POLYMETHACRYLATE (300000 MPA.S) (UNII: 2B25A5KAD6) SHEA BUTTER (UNII: K49155WL9Y) ANGELICA ROOT OIL (UNII: B25G881UOX) LEVOMENOL (UNII: 24WE03BX2T) PENTYLENE GLYCOL (UNII: 50C1307PZG) BERGAMOT OIL (UNII: 39W1PKE3JI) OAT BRAN (UNII: KQX236OK4U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) BLACK OLIVE (UNII: 2M6QWV94OC) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) PRUNUS PERSICA FLOWER (UNII: 19GWB0JENH) REHMANNIA GLUTINOSA ROOT (UNII: 1BEM3U6LQQ) WATER (UNII: 059QF0KO0R) ESCIN (UNII: RUU8G67GQM) SUCROSE STEARATE (UNII: 274KW0O50M) DIMETHICONE (UNII: 92RU3N3Y1O) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PUNICA GRANATUM SEED (UNII: 7294Z34NS7) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83782-101-01 50 g in 1 TANK; Type 0: Not a Combination Product 11/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 11/01/2023 Labeler - Meizhuangzhiku Guangzhou Technology Co.,Ltd (554501763) Registrant - Meizhuangzhiku Guangzhou Technology Co.,Ltd (554501763) Establishment Name Address ID/FEI Business Operations Meizhuangzhiku Guangzhou Technology Co.,Ltd 554501763 manufacture(83782-101)