Label: LEVOTHYROXINE SODIUM injection, solution
- NDC Code(s): 63323-885-10, 63323-890-10, 63323-895-10
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 17, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LEVOTHYROXINE SODIUM INJECTION safely and effectively. See full prescribing information for LEVOTHYROXINE SODIUM ...
-
Table of ContentsTable of Contents
- BOXED WARNING (What is this?)
-
1 INDICATIONS AND USAGE Levothyroxine Sodium Injection is indicated for the treatment of myxedema coma. Limitations of Use: Not recommended as a substitute for oral levothyroxine sodium because the relative ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Dosage - • Consider the age, general physical condition, cardiac risk factors, and clinical severity of myxedema and duration of myxedema symptoms when determining the starting and ...
-
3 DOSAGE FORMS AND STRENGTHS Levothyroxine Sodium Injection is clear, colorless solution supplied as: • 100 mcg per 5 mL (20 mcg per mL) single-dose vial - • 200 mcg per 5 mL (40 mcg per mL) single-dose vial - • 500 mcg per 5 ...
-
4 CONTRAINDICATIONS Uncorrected adrenal insufficiency [see Warnings and Precautions (5.2)]
-
5 WARNINGS AND PRECAUTIONS 5.1 Cardiac Adverse Reactions in the Elderly and in Patients with Underlying Cardiovascular Disease - Overtreatment with Levothyroxine Sodium Injection may cause arrhythmias, tachycardia ...
-
6 ADVERSE REACTIONS Adverse reactions associated with levothyroxine are primarily those of hyperthyroidism due to therapeutic overdosage [see Warnings and Precautions (5), Overdosage (10)]. They include the ...
-
7 DRUG INTERACTIONS 7.1 Drugs Known to Affect Thyroid Hormone Pharmacokinetics - Many drugs affect thyroid hormone pharmacokinetics and metabolism (e.g., synthesis, secretion, catabolism, protein binding, and ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There is no available data with use of Levothyroxine Sodium Injection in pregnant women. The clinical data in pregnant women treated with oral levothyroxine to ...
-
10 OVERDOSAGE The signs and symptoms of overdosage are those of hyperthyroidism [see Warnings and Precautions (5) and Adverse Reactions (6)]. In addition, confusion and disorientation may occur. Cerebral ...
-
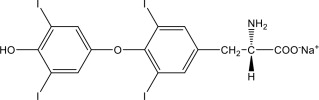
11 DESCRIPTION Levothyroxine Sodium Injection contains synthetic crystalline levothyroxine (T4) in sodium salt form. Levothyroxine sodium has an empirical formula of C15H10I4NNaO4, a molecular weight of 798.85 ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Thyroid hormones exert their physiologic actions through control of DNA transcription and protein synthesis. Triiodothyronine (T3) and levothyroxine (T4) diffuse into ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies have not been performed to evaluate the carcinogenic potential, mutagenic potential or effects on fertility of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Levothyroxine Sodium Injection is a clear, colorless solution available as follows: Product Code - Unit of Sale - Strength - Each - 885110 - NDC 63323-885-10 - Individually ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY – Levothyroxine Sodium Injection 5 mL Vial Label - NDC 63323-885-10 - Levothyroxine Sodium - Injection - 100 mcg per 5 mL - (20 mcg per mL) For intravenous use ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY – Levothyroxine Sodium Injection 5 mL Carton Panel - NDC 63323-885-10 - Levothyroxine Sodium - Injection - 100 mcg per 5 mL - (20 mcg per mL) For intravenous use ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY – Levothyroxine Sodium Injection 5 mL Vial Label - NDC 63323-895-10 - Levothyroxine Sodium - Injection - 500 mcg per 5 mL - (100 mcg per mL) For intravenous use ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY – Levothyroxine Sodium Injection 5 mL Carton Panel - NDC 63323-895-10 - Levothyroxine Sodium - Injection - 500 mcg per 5 mL - (100 mcg per mL) For intravenous use ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY – Levothyroxine Sodium Injection 5 mL Vial Label - NDC 63323-890-10 - Levothyroxine Sodium - Injection - 200 mcg per 5 mL - (40 mcg per mL) For intravenous use ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY – Levothyroxine Sodium Injection 5 mL Carton Panel - NDC 63323-890-10 - Levothyroxine Sodium - Injection - 200 mcg per 5 mL - (40 mcg per mL) For intravenous use ...
-
INGREDIENTS AND APPEARANCEProduct Information