Label: ETHAMBUTOL HYDROCHLORIDE tablet, film coated
- NDC Code(s): 42806-101-01, 42806-102-01
- Packager: Epic Pharma, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
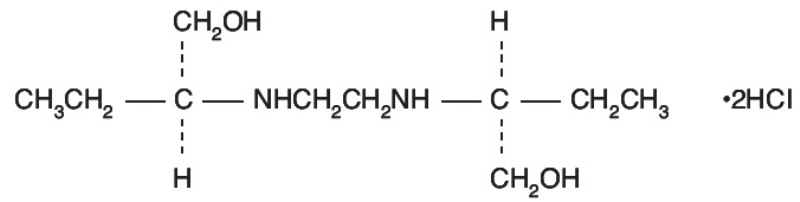
Ethambutol hydrochloride is an oral chemotherapeutic agent which is specifically effective against actively growing microorganisms of the genus Mycobacterium, including M. tuberculosis. Ethambutol ...
-
CLINICAL PHARMACOLOGY Ethambutol hydrochloride following a single oral dose of 25 mg/kg of body weight, attains a peak of 2 to 5 mcg/mL in serum 2 to 4 hours after administration. When the drug is administered daily ...
-
INDICATIONS AND USAGE Ethambutol Hydrochloride Tablets, USP are indicated for the treatment of pulmonary tuberculosis. It should not be used as the sole antituberculous drug, but should be used in conjunction with at ...
-
CONTRAINDICATIONS Ethambutol hydrochloride is contraindicated in patients who are known to be hypersensitive to this drug. It is also contraindicated in patients with known optic neuritis unless clinical judgment ...
-
WARNINGS Ethambutol hydrochloride may produce decreases in visual acuity which appear to be due to optic neuritis. This effect may be related to dose and duration of treatment. This effect is generally ...
-
PRECAUTIONS Ethambutol hydrochloride is not recommended for use in pediatric patients under 13 years of age since safe conditions for use have not been established. Patients with decreased renal function ...
-
ADVERSE REACTIONS Ethambutol hydrochloride may produce decreases in visual acuity, including irreversible blindness, which appear to be due to optic neuritis. Optic neuropathy including optic neuritis or ...
-
DOSAGE AND ADMINISTRATION Ethambutol hydrochloride should not be used alone, in initial treatment or in retreatment. Ethambutol hydrochloride should be administered on a once every 24-hour basis only. Absorption is not ...
-
HOW SUPPLIED Ethambutol Hydrochloride Tablets USP, 100 mg: round, white, film-coated tablets, debossed “VP” on one side and “11” on the other side. • NDC 42806-101-01 Bottles of 100 tablets - Ethambutol ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 100 mg 100ct

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 400 mg 100ct

-
INGREDIENTS AND APPEARANCEProduct Information