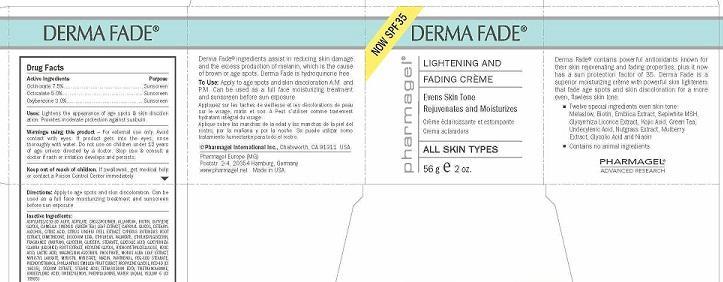
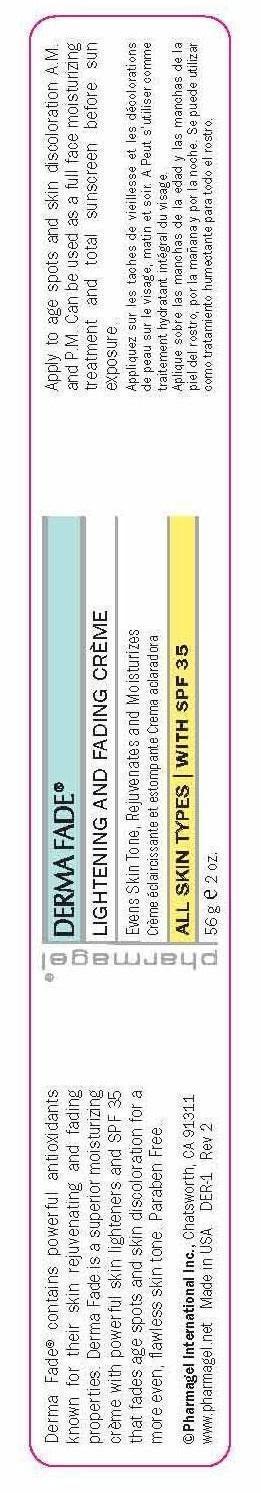
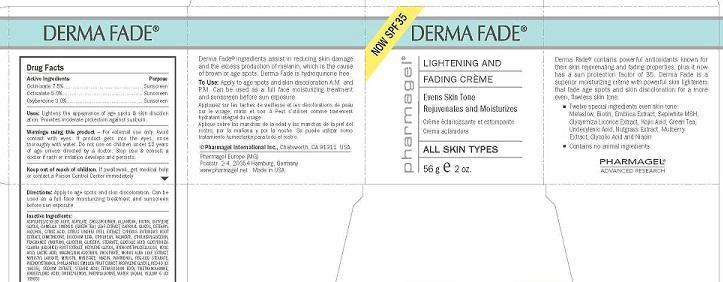
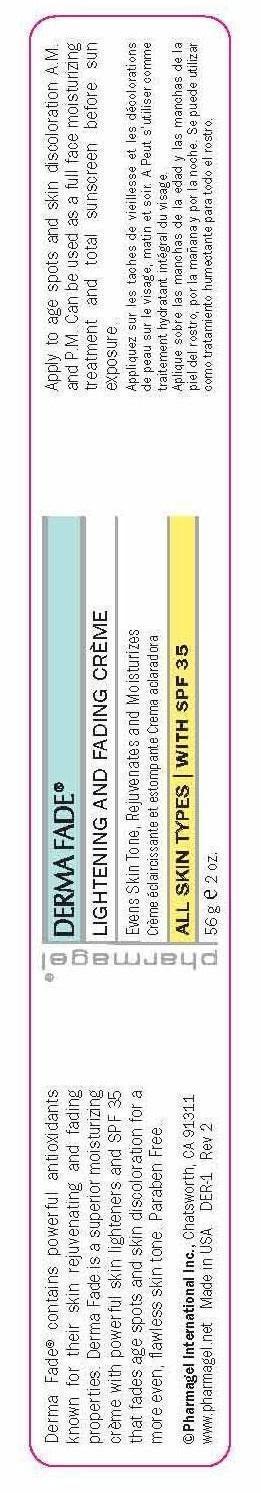
Label: DERMA FADE- octinoxate, octisalate, oxybenzone cream
- NDC Code(s): 67879-301-11, 67879-301-51
- Packager: PHARMAGEL INTERNATIONAL INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
-
INACTIVE INGREDIENTS:
ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, ALLANTOIN, BIOTIN, BUTYLENE GLYCOL, CAMELLIA SINENSIS (GREEN TEA) LEAF EXTRACT, CAPRYLYL GLYCOL, CETEARYL ALCOHOL, CITRIC ACID, CITRUS UNSHIU PEEL EXTRACT, CYPERUS ROTUNDUS ROOT EXTRACT, DIMETHICONE, DISODIUM EDTA, ETHYLHEXYL PALMITATE, ETHYLHEXYLGLYCERIN, FRAGRANCE (PARFUM), GLYCERIN, GLYCERYL STEARATE, GLYCOLIC ACID, GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT, HEXYLENE GLYCOL, HYDROXYETHYLCELLULOSE, KOJIC ACID, LACTIC ACID, MAGNESIUM ASCORBYL PHOSPHATE, MORUS ALBA LEAF EXTRACT, MYRISTYL LAURATE, MYRISTYL MYRISTATE, NIACIN, PANTHENOL, PEG-100 STEARATE, PHENOXYETHANOL, PHYLLANTHUS EMBLICA FRUIT EXRACT, PROPYLENE GLYCOL, RED 40 (CI 16035), SODIUM CITRATE, STEARIC ACID, TETRASODIUM EDTA, TRIETHANOLAMINE, UNDECYLENIC ACID, UNDECYLENOYL PHENYLALANINE, WATER (AQUA), YELLOW 6 (CI 15985)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DERMA FADE
octinoxate, octisalate, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67879-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 3 g in 100 g Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) ALLANTOIN (UNII: 344S277G0Z) BIOTIN (UNII: 6SO6U10H04) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) TANGERINE PEEL (UNII: JU3D414057) CYPERUS ROTUNDUS TUBER (UNII: 4B51SRR959) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLHEXYL PALMITATE (UNII: 2865993309) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCOLIC ACID (UNII: 0WT12SX38S) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) HEXYLENE GLYCOL (UNII: KEH0A3F75J) HYDROXYETHYL CELLULOSE (3000 MPA.S AT 1%) (UNII: 7Q6P4JN1QT) KOJIC ACID (UNII: 6K23F1TT52) LACTIC ACID (UNII: 33X04XA5AT) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) MORUS ALBA LEAF (UNII: M8YIA49Q2P) MYRISTYL LAURATE (UNII: 58U0NZN2BT) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) NIACIN (UNII: 2679MF687A) PANTHENOL (UNII: WV9CM0O67Z) PEG-100 STEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYLLANTHUS EMBLICA FRUIT (UNII: YLX4CW2576) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FD&C RED NO. 40 (UNII: WZB9127XOA) SODIUM CITRATE (UNII: 1Q73Q2JULR) STEARIC ACID (UNII: 4ELV7Z65AP) EDETATE SODIUM (UNII: MP1J8420LU) TROLAMINE (UNII: 9O3K93S3TK) UNDECYLENIC ACID (UNII: K3D86KJ24N) UNDECYLENOYL GLYCINE (UNII: 4D20464K2J) WATER (UNII: 059QF0KO0R) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67879-301-51 1 in 1 BOX 06/18/2015 1 NDC:67879-301-11 56 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/18/2015 Labeler - PHARMAGEL INTERNATIONAL INC (603215182)