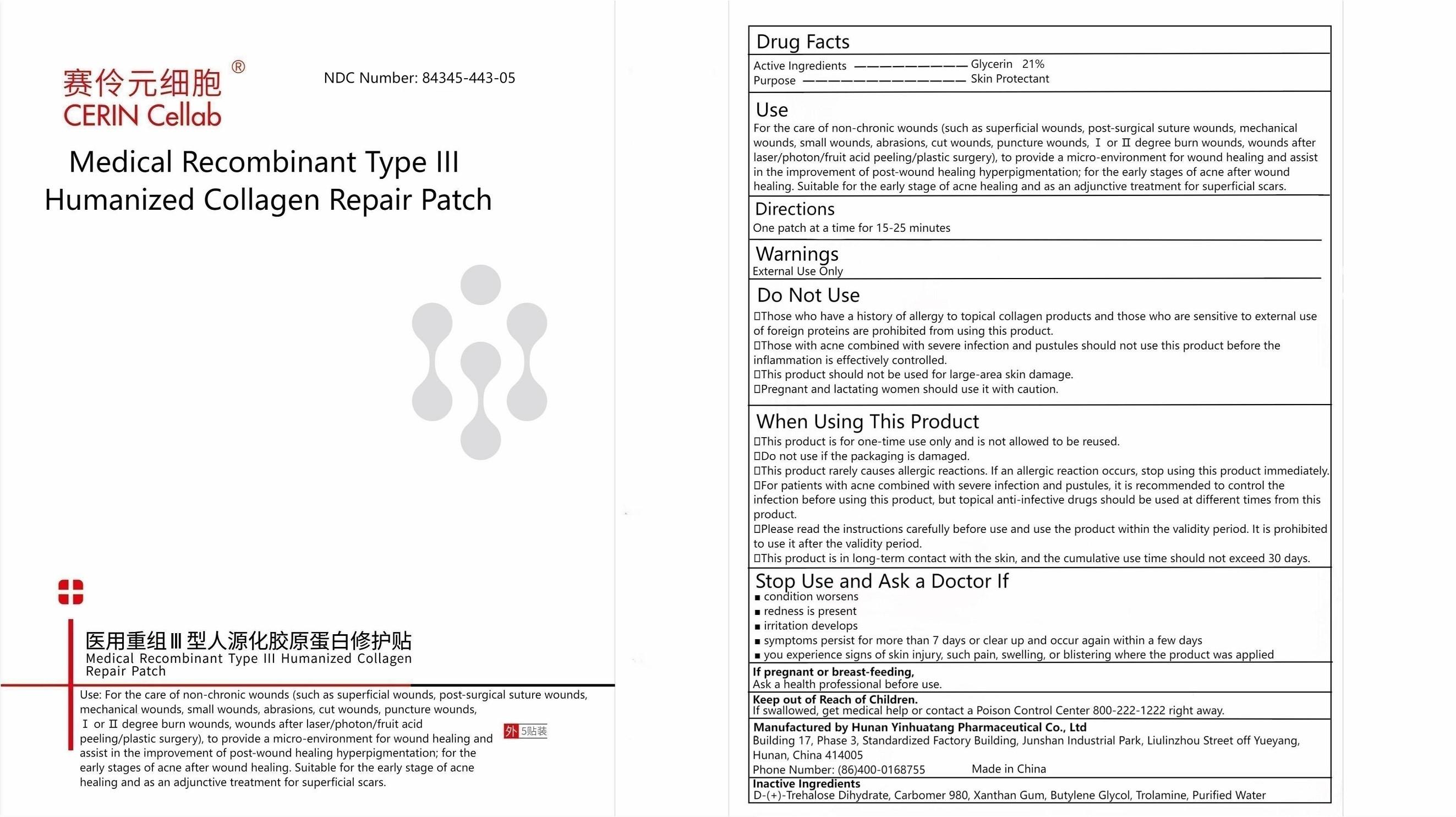
Label: MEDICAL RECOMBINANT TYPE III HUMANIZED COLLAGEN REPAIR PATCH patch
- NDC Code(s): 84345-443-05
- Packager: Hunan Yinhuatang Pharmaceutical Technology Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
-
Use
For the care of non-chronic wounds (such as superficial wounds, post-surgical suture wounds, mechanical wounds, small wounds, abrasions, cut wounds, puncture wounds, Ⅰ or Ⅱ degree burn wounds, wounds after laser/photon/fruit acid peeling/plastic surgery), to provide a micro-environment for wound healing and assist in the improvement of post-wound healing hyperpigmentation; for the early stages of acne after wound healing. Suitable for the early stage of acne healing and as an adjunctive treatment for superficial scars.
- Directions
- Warnings
-
Do Not Use
- Those who have a history of allergy to topical collagen products and those who are sensitive to external use of foreign proteins are prohibited from using this product.
- Those with acne combined with severe infection and pustules should not use this product before the inflammation is effectively controlled.
- This product should not be used for large-area skin damage.
- Pregnant and lactating women should use it with caution.
-
When Using This Product
- This product is for one-time use only and is not allowed to be reused.
- Do not use if the packaging is damaged.
- This product rarely causes allergic reactions. If an allergic reaction occurs, stop using this product immediately.
- For patients with acne combined with severe infection and pustules, it is recommended to control the infection before using this product, but topical anti-infective drugs should be used at different times from this product.
- Please read the instructions carefully before use and use the product within the validity period. It is prohibited to use it after the validity period.
- This product is in long-term contact with the skin, and the cumulative use time should not exceed 30 days.
- Stop Use and Ask A Doctor If
- If pregnant or breast-feeding,
- Keep out of Reach of Children.
- Manufactured by Hunan Yinhuatang Pharmaceutical Co., Ltd. Building 17, Phase 3, Standardized Factory Building, Junshan Industrial Park, Liulinzhou Street Off Yueyang, Hunan, China 414005Phone Number: (86)400-0168755
- Inactive Ingredients
- Package Label
-
INGREDIENTS AND APPEARANCE
MEDICAL RECOMBINANT TYPE III HUMANIZED COLLAGEN REPAIR PATCH
medical recombinant type iii humanized collagen repair patch patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84345-443 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 21 g in 100 g Inactive Ingredients Ingredient Name Strength TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) CARBOMER 980 (UNII: 4Q93RCW27E) XANTHAN GUM (UNII: TTV12P4NEE) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84345-443-05 5 in 1 PACKAGE 07/22/2024 1 30 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 07/19/2024 Labeler - Hunan Yinhuatang Pharmaceutical Technology Co., Ltd (700229500)