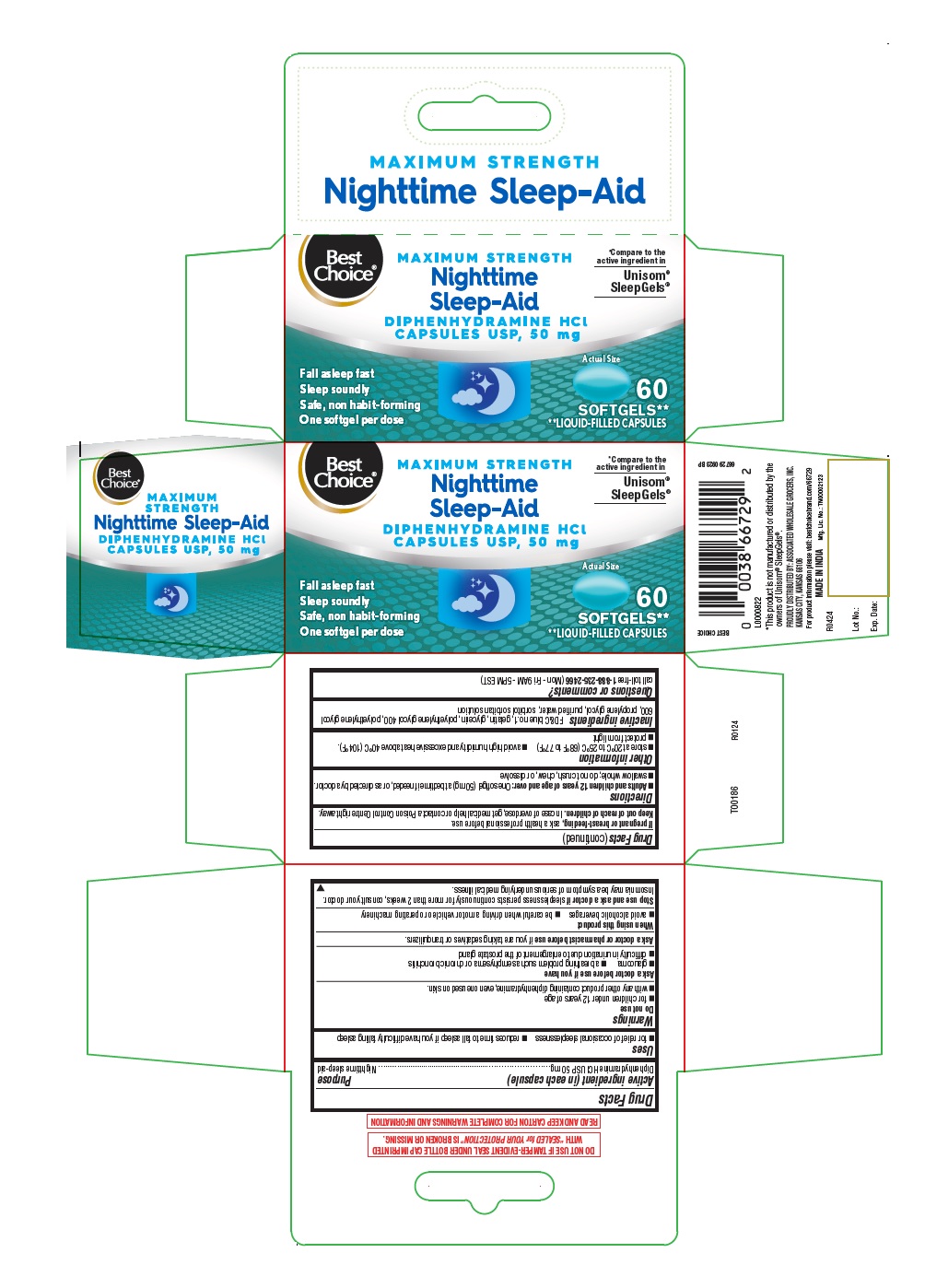
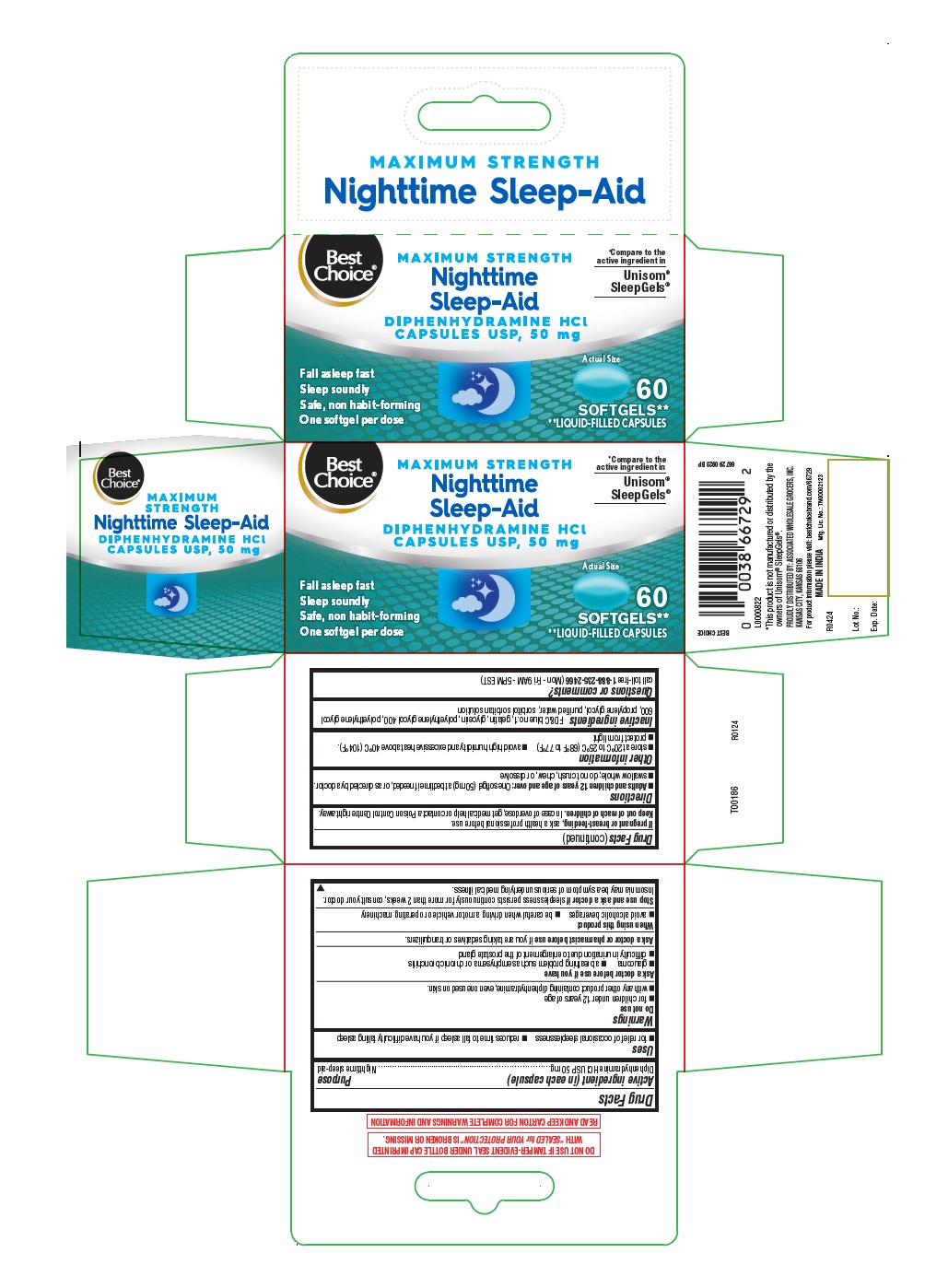
Label: NIGHTTIME SLEEP-AID- diphenhydramine hcl capsule, liquid filled
- NDC Code(s): 63941-604-17
- Packager: VALU MERCHANDISERS COMPANY
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each capsule)
- Purpose
- Uses
- Warnings
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast- feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
DO NOT USE IF TAMPER-EVIDENT SEAL UNDER BOTTLE CAP IMPRINTED WITH “SEALED for YOUR PROTECTION” IS BROKEN OR MISSING.
READ AND KEEP CARTON FOR COMPLETE WARNINGS AND INFORMATION
BEST CHOICE 66729 0923 BP
T00186 R0124
*This product is not manufactured or distributed by the owners of Unisom® SleepGels®.
PROUDLY DISTRIBUTED BY: ASSOCIATED WHOLESALE GROCERS, INC. KANSAS CITY, KANSAS 66106
For product information please visit: bestchoicebrand.com/66729
MADE IN INDIA
Mfg. Lic. No.: TN00002123
L0000822
R0424
Lot No.:
Exp. Date:
- 60's count
-
INGREDIENTS AND APPEARANCE
NIGHTTIME SLEEP-AID
diphenhydramine hcl capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63941-604 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 50 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 600 (UNII: NL4J9F21N9) SORBITAN (UNII: 6O92ICV9RU) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBITOL (UNII: 506T60A25R) Product Characteristics Color blue Score no score Shape OVAL Size 13mm Flavor Imprint Code B50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63941-604-17 1 in 1 CARTON 05/01/2024 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M010 05/01/2024 Labeler - VALU MERCHANDISERS COMPANY (868703513) Registrant - Bionpharma Inc. (079637826) Establishment Name Address ID/FEI Business Operations SOFTGEL HEALTHCARE PRIVATE LIMITED 675584180 manufacture(63941-604)