Label: ANTACID CHILDRENS- calcium carbonate tablet, chewable
- NDC Code(s): 59779-596-08
- Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
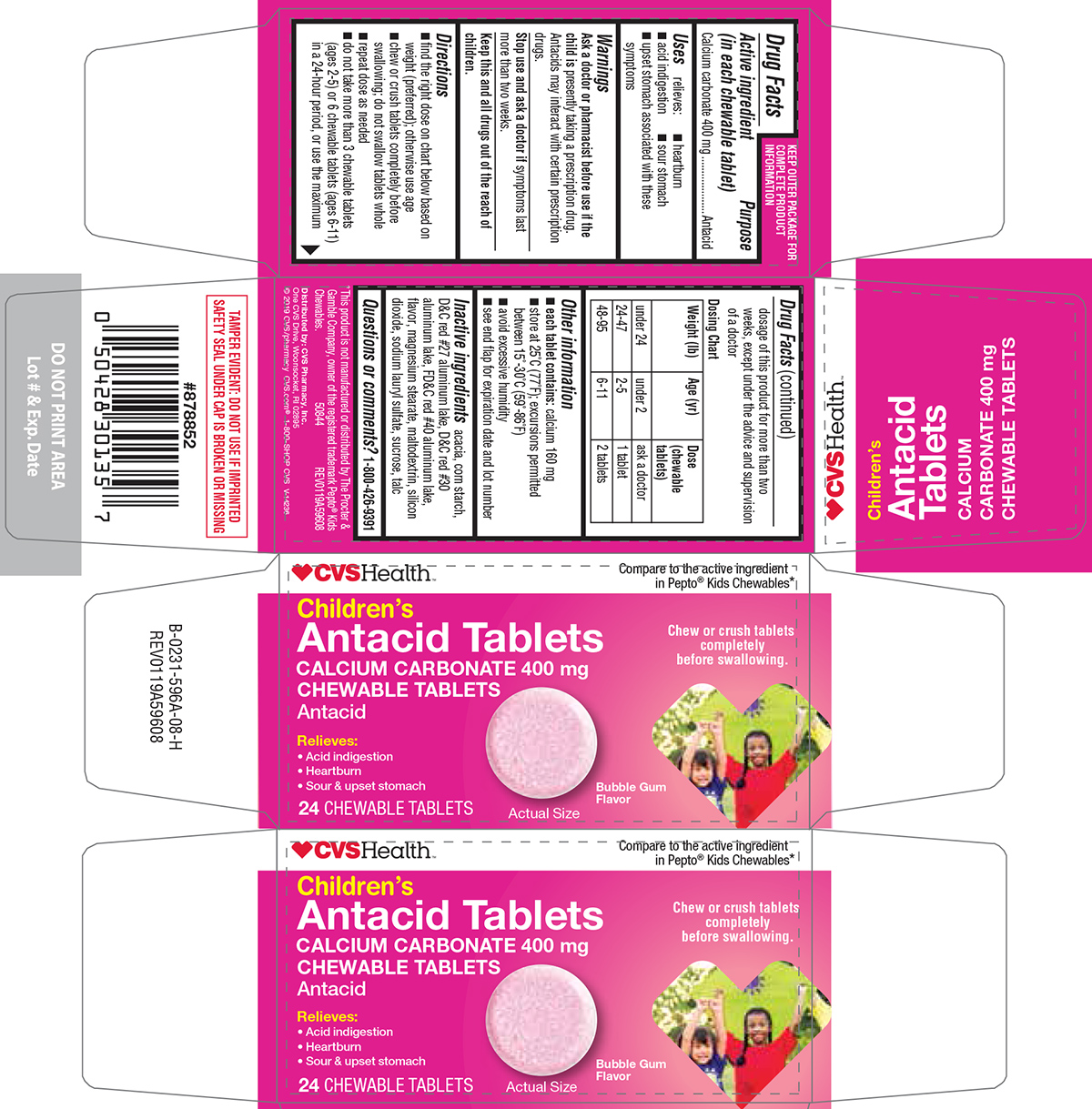
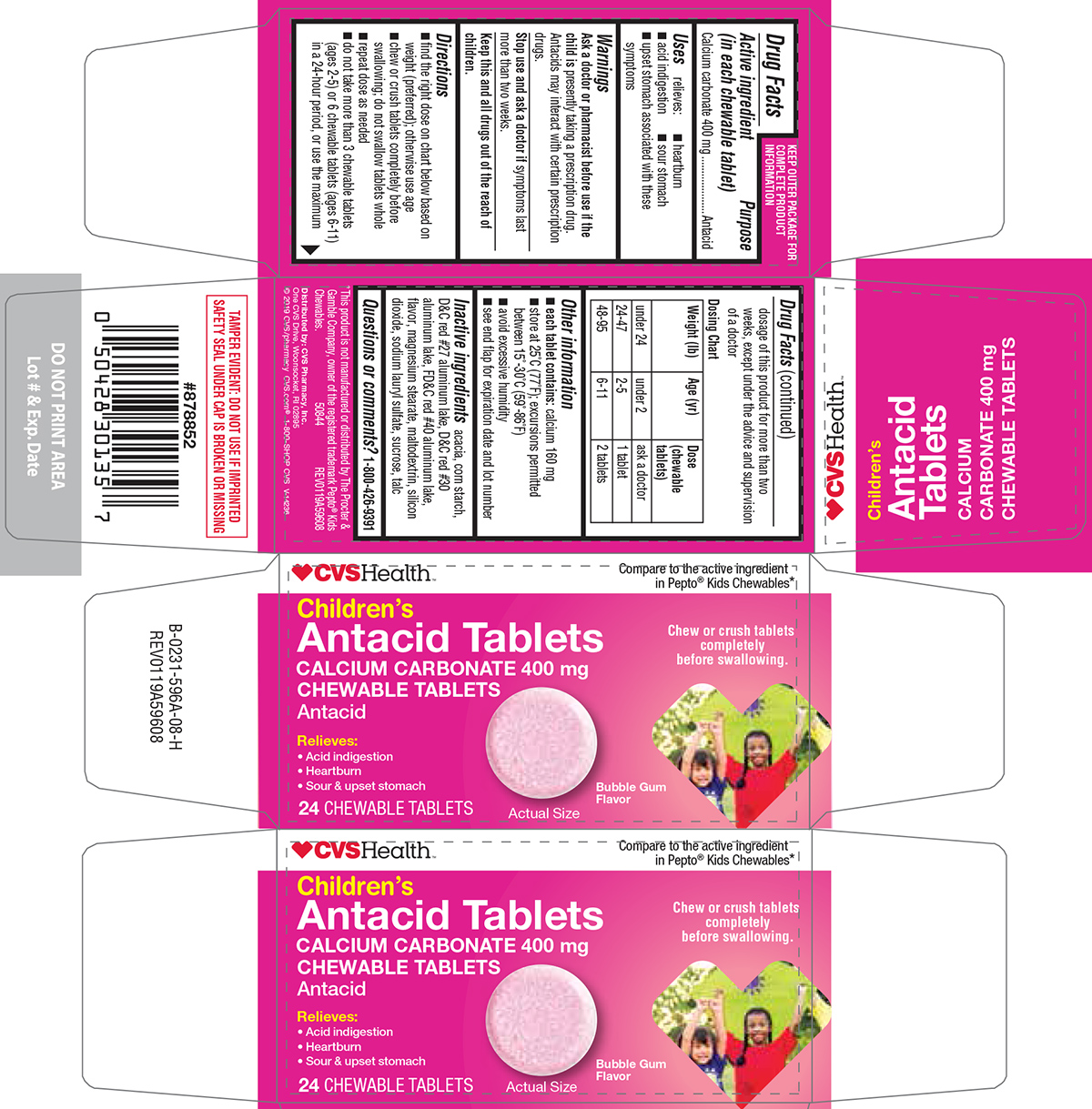
- Active ingredient (in each chewable tablet)
- Purpose
- Uses
- Warnings
-
Directions
- find the right dose on chart below based on weight (preferred); otherwise use age
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- repeat dose as needed
- do not take more than 3 chewable tablets (ages 2-5) or 6 chewable tablets (ages 6-11) in a 24-hour period, or use the maximum dosage of this product for more than two weeks, except under the advice and supervision of a doctor
Dosing Chart
Weight (lb) Age (yr) Dose (chewable tablets) under 24 under 2 ask a doctor 24-47 2-5 1 tablet 48-95 6-11 2 tablets - Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
♥CVSHealth™
Compare to the active ingredient
in Pepto® Kids Chewables*Children's
Antacid Tablets
CALCIUM CARBONATE 400 mg
CHEWABLE TABLETS
AntacidRelieves:
• Acid indigestion
• Heartburn
• Sour & upset stomachChew or crush tablets
completely
before swallowing.24 CHEWABLE TABLETS
Bubble Gum
FlavorActual Size
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING*This product is not manufactured or distributed by The Procter &
Gamble Company, owner of the registered trademark Pepto® Kids
Chewables. 50844 REV0119A59608
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2019 CVS/pharmacy
CVS.com® 1-800-SHOP CVSV-14236
CVS 44-596A
-
INGREDIENTS AND APPEARANCE
ANTACID CHILDRENS
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-596 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 400 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) STARCH, CORN (UNII: O8232NY3SJ) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) D&C RED NO. 30 ALUMINUM LAKE (UNII: GE75M6YV5W) FD&C RED NO. 40 ALUMINUM LAKE (UNII: 6T47AS764T) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color pink Score no score Shape ROUND Size 16mm Flavor BUBBLE GUM Imprint Code 44;596 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-596-08 1 in 1 CARTON 08/01/2018 1 24 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 08/01/2018 Labeler - CVS Pharmacy (062312574) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(59779-596) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(59779-596) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(59779-596) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(59779-596)