Label: BISOPROLOL FUMARATE tablet
- NDC Code(s): 43547-616-03, 43547-616-10, 43547-616-50, 43547-617-03, view more
- Packager: Solco Healthcare US, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
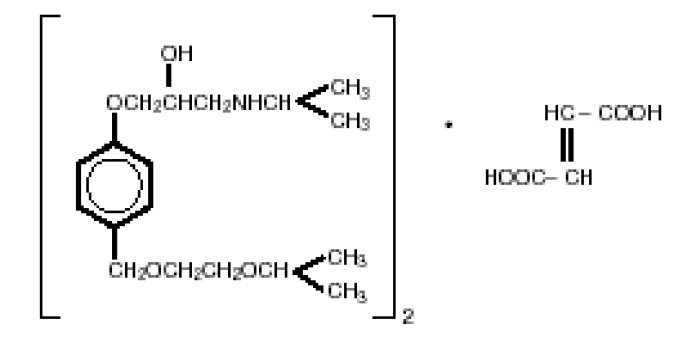
DESCRIPTIONBisoprolol fumarate tablets are a synthetic, beta1-selective (cardioselective) adrenoceptor blocking agent. The chemical name for bisoprolol fumarate is ...
-
CLINICAL PHARMACOLOGY Bisoprolol fumarate tablets are a beta1-selective (cardioselective) adrenoceptor blocking agent without significant membrane stabilizing activity or intrinsic sympathomimetic activity in its ...
-
CLINICAL STUDIES In two randomized double-blind placebo-controlled trials conducted in the U.S., reductions in systolic and diastolic blood pressure and heart rate 24 hours after dosing in patients with ...
-
INDICATIONS AND USAGE Bisoprolol fumarate tablets are indicated in the management of hypertension. It may be used alone or in combination with other antihypertensive agents.
-
CONTRAINDICATIONS Bisoprolol fumarate tablets are contraindicated in patients with cardiogenic shock, overt cardiac failure, second or third degree AV block, and marked sinus bradycardia.
-
WARNINGS Click here to enter Warnings - Cardiac Failure - Sympathetic stimulation is a vital component supporting circulatory function in the setting of congestive heart failure, and beta-blockade may ...
-
PRECAUTIONS Impaired Renal or Hepatic Function - Use caution in adjusting the dose of bisoprolol fumarate tablets in patients with renal or hepatic impairment (see CLINICAL PHARMACOLOGY). Drug ...
-
ADVERSE REACTIONS Safety data are available in more than 30,000 patients or volunteers. Frequency estimates and rates of withdrawal of therapy for adverse events were derived from two U.S. placebo-controlled ...
-
LABORATORY ABNORMALITIES In clinical trials, the most frequently reported laboratory change was an increase in serum triglycerides, but this was not a consistent finding. Sporadic liver test abnormalities have been ...
-
OVERDOSAGE The most common signs expected with overdosage of a beta-blocker are bradycardia, hypotension, congestive heart failure, bronchospasm, and hypoglycemia. To date, a few cases of overdose (maximum ...
-
DOSAGE AND ADMINISTRATION The dose of bisoprolol fumarate tablets must be individualized to the needs of the patient. The usual starting dose is 5 mg once daily. In some patients, 2.5 mg may be an appropriate starting dose ...
-
HOW SUPPLIED Bisoprolol fumarate tablets, USP, are supplied as 5 mg and 10 mg tablets. The 5 mg tablet is pink color coated tablet, capsule shaped, convex scored tablets debossed with “6|6” on one side and “S ...
-
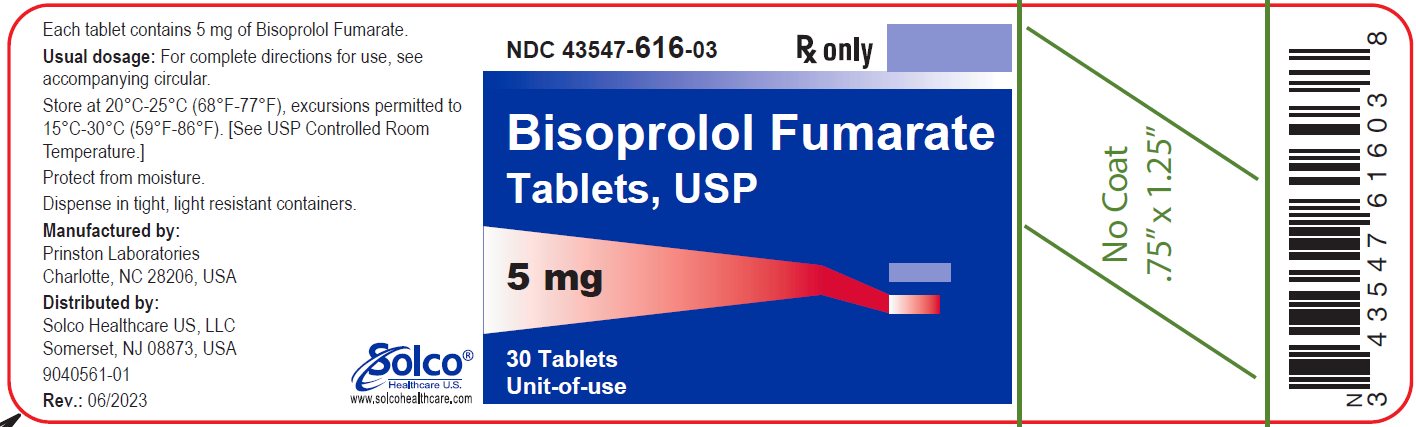
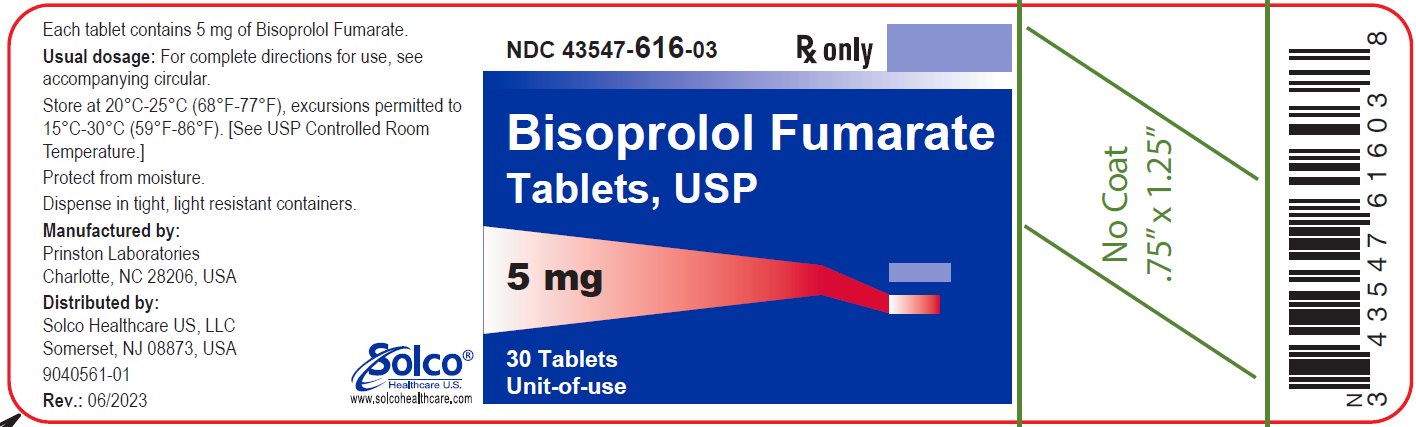
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL 43547-616-03 - Rx Only - Bisoprolol Fumarate Tablets, USP - 5 mg 30 Tablets - Unit-of-use - Each tablet contains 5 mg of bisoprolol Fumarate. Usual dosage: See package insert for complete ...
-
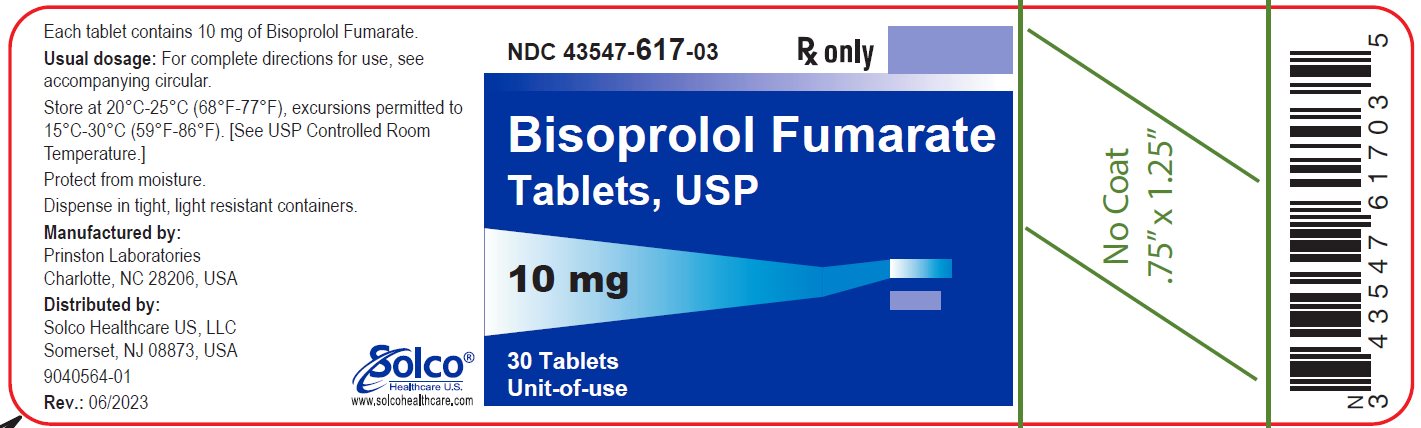
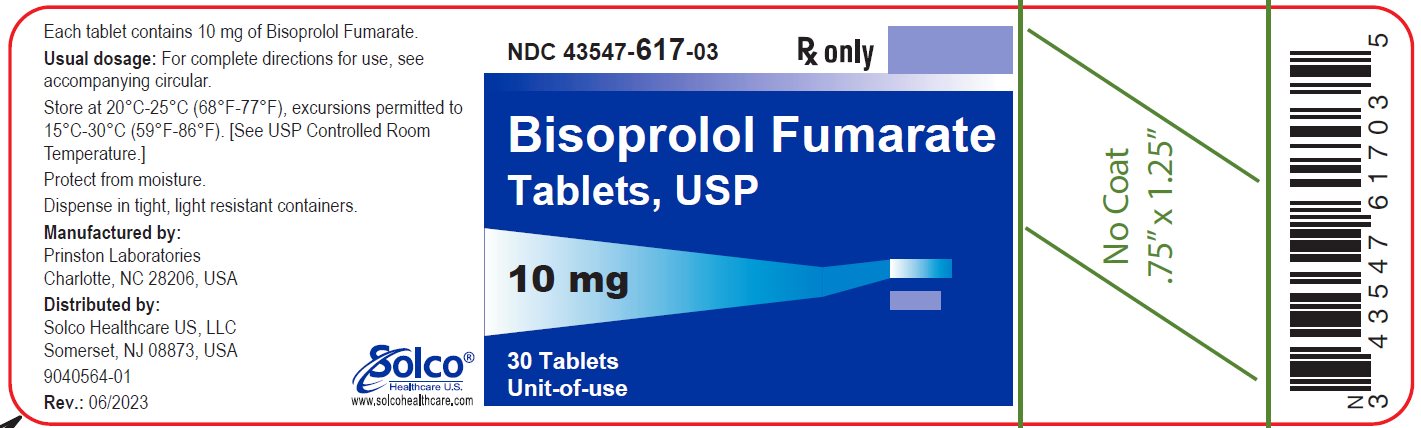
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL 43547-617-03 - Rx Only - Bisoprolol Fumarate Tablets, USP - 10 mg 30 Tablets - Unit-of-use - Each tablet contains 10 mg of bisoprolol Fumarate. Usual dosage: See package insert for complete ...
-
INGREDIENTS AND APPEARANCEProduct Information