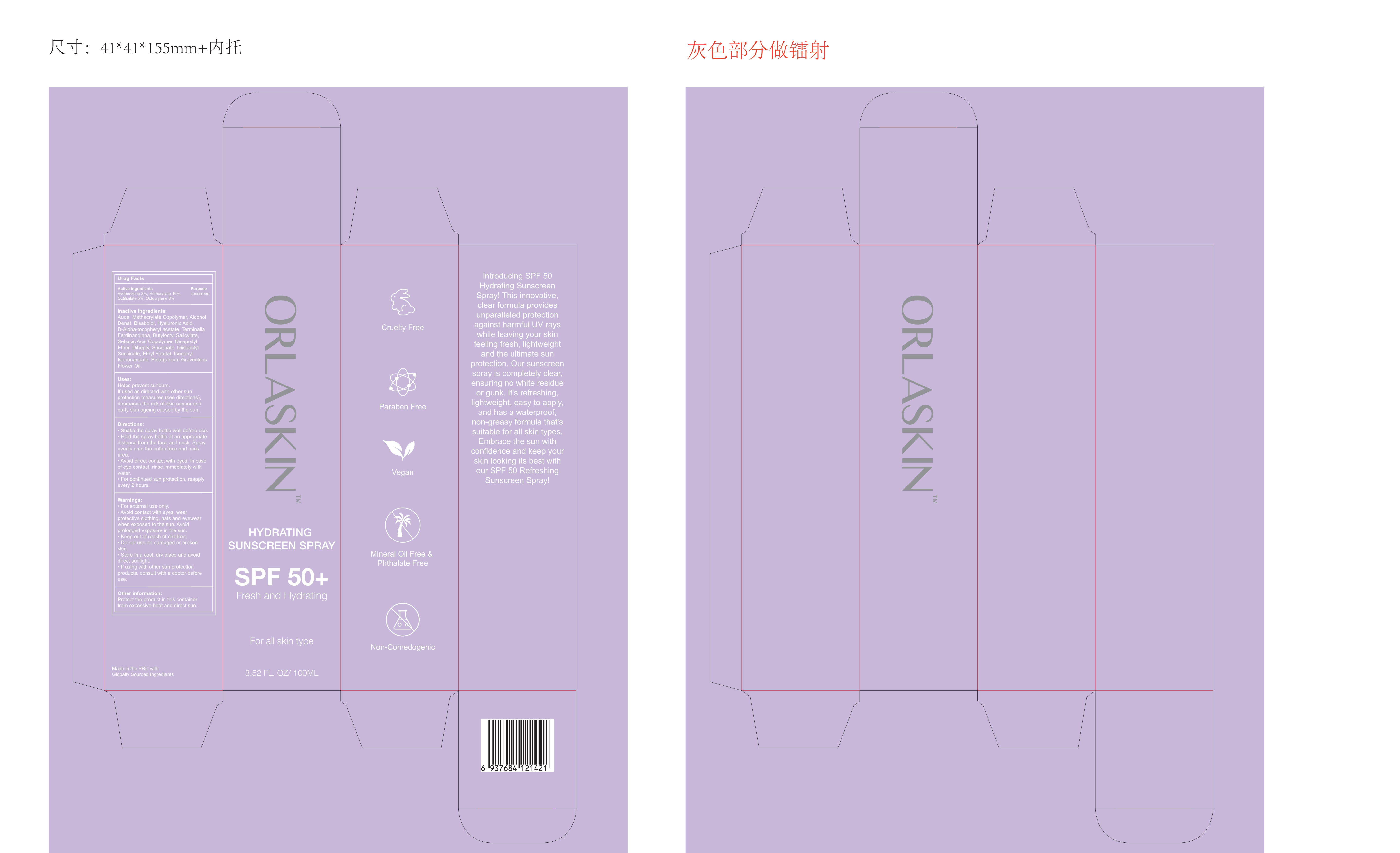
Label: ORLASKIN HYDRATING SUNSCREEN spray
- NDC Code(s): 83447-003-01
- Packager: Guangzhou Fantesy Biotechnology Co.,Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- Uses
-
Warnings
For external use only.
Avoid contact with eyes , wear protective clothing , hats and eyewear when exposed to the sun . Avoid prolonged exposure in the sun.
Keep out of reach of children.
Do not use on damaged or brokenskin.
Store in a cool , dry place and avoiddirect sunlight.
If using with other sun protection products , consult with a doctor before use.
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Shake the spray bottle well before use.
Hold the spray bottle at an appropriate distance from the face and neck . Spray evenly onto the entire face and neck area.
Avoid direct contact with eves . In case of eye contact , rinse immediately with water.
For continued sun protection , reapply every 2 hours
- Other information
-
Inactive Ingredients
Auqa , Methacrylate Copolymer , Alcohol Denat , Bisabolol , Hvaluronic Acid, D-alpha-tocopheryl acetate , Terminalia Ferdinandiana , Butyloctyl Salicylate, Sebacic Acid Copolymer , Dicaprylyl Ether , Diheptyl Succinate , Diisooctyl Succinate , Ethyl Ferulat , Isononyl sononanoate , Pelargonium Graveolens Flower Oil
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ORLASKIN HYDRATING SUNSCREEN
orlaskin hydrating sunscreen sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83447-003 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 8 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength HYALURONIC ACID (UNII: S270N0TRQY) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) KAKADU PLUM (UNII: 0ZQ1D2FDLI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER (2000 MPA.S) (UNII: N7YC58165T) WATER (UNII: 059QF0KO0R) ETHYL ACRYLATE AND METHYL METHACRYLATE COPOLYMER (2:1; 750000 MW) (UNII: P2OM2Q86BI) ALCOHOL (UNII: 3K9958V90M) LEVOMENOL (UNII: 24WE03BX2T) DICAPRYLYL ETHER (UNII: 77JZM5516Z) DIHEPTYL SUCCINATE (UNII: 057N7SS26Y) ETHYL FERULATE (UNII: 5B8915UELW) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) PELARGONIUM GRAVEOLENS FLOWER OIL (UNII: 3K0J1S7QGC) DOCUSATE SODIUM (UNII: F05Q2T2JA0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83447-003-01 100 mL in 1 BOX; Type 0: Not a Combination Product 10/19/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/19/2023 Labeler - Guangzhou Fantesy Biotechnology Co.,Ltd (619047084) Establishment Name Address ID/FEI Business Operations Guangzhou Fantesy Biotechnology Co.,Ltd 619047084 manufacture(83447-003)