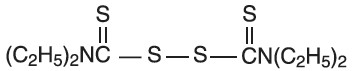Label: DISULFIRAM tablet
- NDC Code(s): 62135-431-30, 62135-431-90, 62135-432-30, 62135-432-90
- Packager: Chartwell RX, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONWARNING - Disulfiram should - never be administered to a patient when he is in a state of alcohol intoxication, or without his full knowledge ...
-
DESCRIPTIONDisulfiram is an alcohol antagonist drug. CHEMICAL NAME: bis(diethylthiocarbamoyl) disulfide. STRUCTURAL FORMULA: C - 10H - 20N ...
-
CLINICAL PHARMACOLOGYDisulfiram produces a sensitivity to alcohol which results in a highly unpleasant reaction when the patient under treatment ingests even small amounts of alcohol. Disulfiram blocks the oxidation ...
-
INDICATIONS AND USAGEDisulfiram is an aid in the management of selected chronic alcohol patients who - want to remain in a state of enforced sobriety so that supportive and psychotherapeutic ...
-
CONTRAINDICATIONSPatients who are receiving or have recently received metronidazole, paraldehyde, alcohol, or alcohol-containing preparations, e.g., cough syrups, tonics and the like, should not be given ...
-
WARNINGSDisulfiram should - never be administered to a patient when he is in a state of alcohol intoxication, or without his full knowledge ...
-
PRECAUTIONSPatients with a history of rubber contact dermatitis should be evaluated for hypersensitivity to thiuram derivatives before receiving disulfiram (see ...
-
Drug InteractionsDisulfiram appears to decrease the rate at which certain drugs are metabolized and therefore may increase the blood levels and the possibility of clinical toxicity of drugs given ...
-
ADVERSE REACTIONS(See - CONTRAINDICATIONS - , WARNINGS - , and ...
-
OVERDOSAGENo specific information is available on the treatment of overdosage with disulfiram. It is recommended that the physician contact the local Poison Control Center.
-
DOSAGE AND ADMINISTRATIONDisulfiram should never be administered until the patient has abstained from alcohol for at least 12 hours. Initial Dosage Schedule - In the first phase of treatment, a ...
-
HOW SUPPLIEDDisulfiram Tablets, USP are available as: 250 mg – Off-white, round, biconvex, unscored tablets, debossed “CE” over “31” on one side and plain on the other side. The tablets are supplied in ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANELDisulfiram Tablets, USP 250 mg - NDC 62135-431-30 - 30 Tablets Label - Disulfiram Tablets, USP 250 mg - NDC 62135-431-90 - 90 Tablets Label - Disulfiram Tablets, USP 500 mg - NDC 62135-432-30 ...
-
INGREDIENTS AND APPEARANCEProduct Information