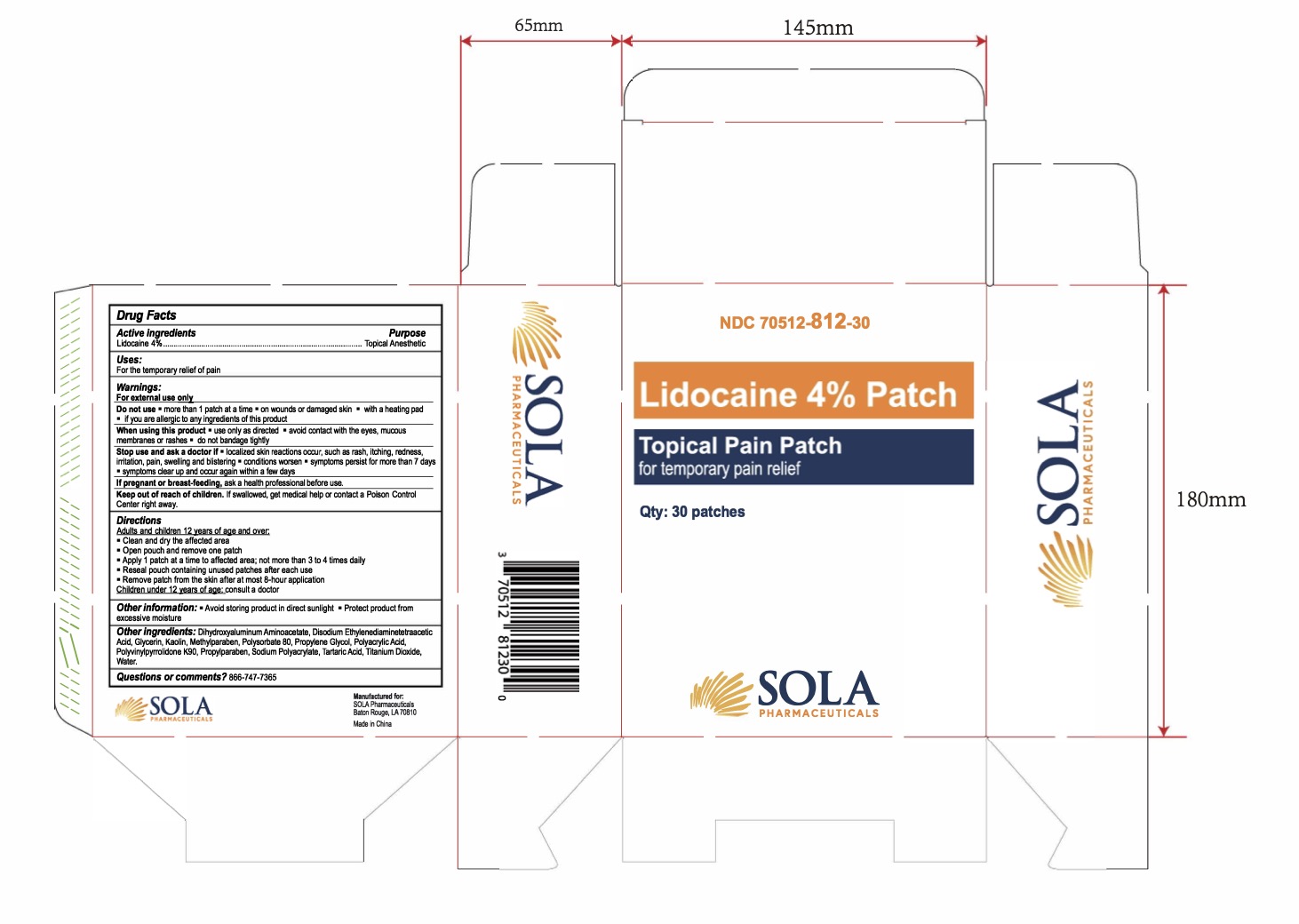
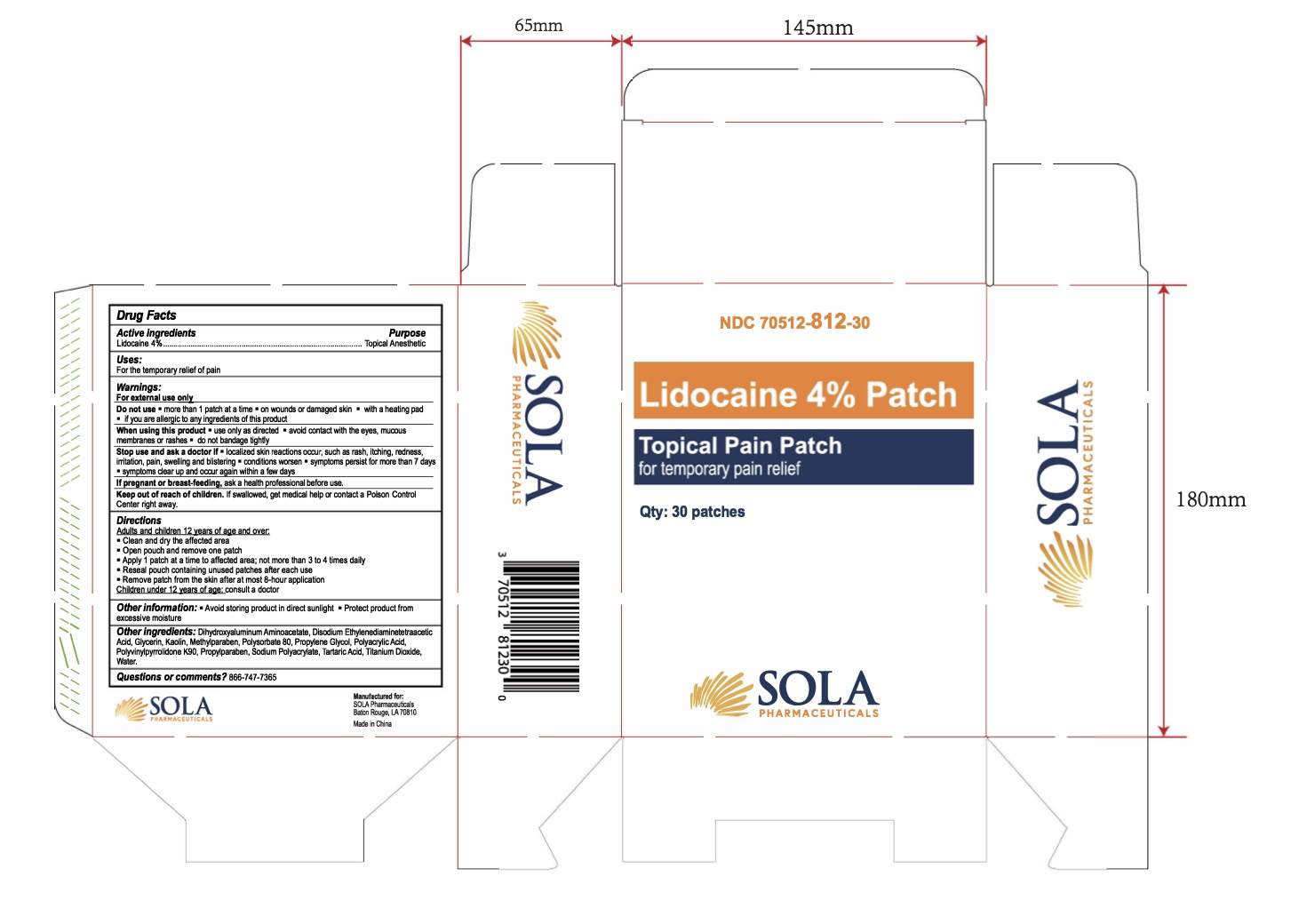
Label: LIDOCAINE 4% PATCH- lidocaine 4% patch
- NDC Code(s): 70512-812-30
- Packager: SOLA Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- DO NOT USE
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Adults and children 12 years of age and over:
- Clean and dry the affected area
- Open pouch and remove one patch
- Apply 1 patch at a time to affected area; not more than 3 to 4 times daily
- Reseal pouch containing unused patches after each use
- Remove patch from the skin after at most 8-hour application
Children under 12 years of age: consult a doctor
- Other Information
- Other Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIDOCAINE 4% PATCH
lidocaine 4% patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70512-812 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 40 mg in 1 g Inactive Ingredients Ingredient Name Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GLYCERIN (UNII: PDC6A3C0OX) DISODIUM ETHYLENEDIAMINEDIACETATE (UNII: EQL53S5L0F) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) KAOLIN (UNII: 24H4NWX5CO) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE K90 (UNII: RDH86HJV5Z) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) TARTARIC ACID (UNII: W4888I119H) Product Characteristics Color Score Shape RECTANGLE Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70512-812-30 30 in 1 BOX 10/12/2023 1 1 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/12/2023 Labeler - SOLA Pharmaceuticals (080121345)