Label: ERY- erythromycin swab
- NDC Code(s): 63629-8648-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 45802-962
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Ery 2% Pads contain erythromycin, USP for topical dermatologic use. Erythromycin is a macrolide antibiotic produced from a strain of Saccaropolyspora erythraea(formerly Streptomyces erythreus). It is a base and readily forms salts with acids.
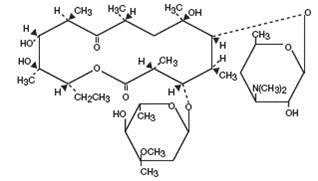
Chemically, erythromycin is C 37H 67NO 13. It has the following structural formula:
The chemical name for erythromycin is (3 R*,4 S*,5 S*,6 R*,7 R*,9 R*,11 R*,12 R*,13 S*,14 R*)-4-[(2,6-Dideoxy-3- C-methyl-3- O-methyl-α-L- ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-β-D- xylo-hexopyranosyl]oxy] oxacyclotetradecane-2,10-dione.
Erythromycin has the molecular weight of 733.94. It is a white or slightly yellow, crystalline powder, slightly soluble in water, soluble in alcohol, in chloroform, and in ether. It is odorless or practically odorless. It has a pH range between 8.0 and 10.5 in a methanol and water solution prepared by diluting 1 volume of a methanol solution, containing 40 mg per mL, with 19 volumes of water.
Each mL of expressible liquid contains 20 mg erythromycin in a base of dehydrated alcohol, propylene glycol and citric acid to adjust pH. Each pledget is filled to contain 0.8 mL of Erythromycin Topical Solution 2%.
-
CLINICAL PHARMACOLOGY
The exact mechanism by which erythromycin reduces lesions of acne vulgaris is not fully known; however, the effect appears to be due in part to the antibacterial activity of the drug.
MICROBIOLOGY
Erythromycin acts by inhibition of protein synthesis in susceptible organisms by reversibly binding to 50S ribosomal subunits, thereby inhibiting translocation of aminoacyl transfer-RNA and inhibiting polypeptide synthesis. Antagonism has been demonstrated in vitrobetween erythromycin, lincomycin, chloramphenicol, and clindamycin.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including erythromycin, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficileis one primary cause of “antibiotic-associated colitis”.
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation and treatment with an antibacterial drug clinically effective against C. difficilecolitis.
-
PRECAUTIONS
General -
For topical use only; not for ophthalmic use. Concomitant topical acne therapy should be used with caution because a possible cumulative irritancy effect may occur, especially with the use of peeling, desquamating, or abrasive agents.
The use of antibiotic agents may be associated with the overgrowth of antibiotic-resistant organisms. If this occurs, discontinue use and take appropriate measures.
Avoid contact with eyes and all mucous membranes.
Information for Patients -
Patients using Ery 2% Pads should receive the following information and instructions:
1. This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes, nose, mouth, and all mucous membranes.
2. This medication should not be used for any disorder other than that for which it was prescribed.
3. Patients should not use any other topical acne medication unless otherwise directed by their physician.
4. Patients should report to their physician any signs of local adverse reactions.
Carcinogenesis, Mutagenesis, Impairment of Fertility -
No animal studies have been performed to evaluate the carcinogenic and mutagenic potential or effects on fertility of topical erythromycin. However, long-term (2-year) oral studies in rats with erythromycin ethylsuccinate and erythromycin base did not provide evidence of tumorigenicity. There was no apparent effect on male or female fertility in rats fed erythromycin (base) at levels up to 0.25% of diet.
Pregnancy:
Teratogenic Effects:
Pregnancy Category B -
There was no evidence of teratogenicity or any other adverse effect on reproduction in female rats fed erythromycin base (up to 0.25% diet) prior to and during mating, during gestation and through weaning of two successive litters.
There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used in pregnancy only if clearly needed. Erythromycin has been reported to cross the placental barrier in humans, but fetal plasma levels are generally low.
Nursing Women -
It is not known whether erythromycin is excreted in human milk after topical application. However, erythromycin is excreted in human milk following oral and parenteral erythromycin administration. Therefore, caution should be exercised when erythromycin is administered to a nursing woman.
-
ADVERSE REACTIONS
The following local adverse reactions have been reported occasionally: peeling, dryness, itching, erythema, and oiliness. Irritation of the eyes and tenderness of the skin have also been reported with topical use of erythromycin. Generalized urticarial reactions, possibly related to the use of erythromycin, which required systemic steroid therapy have been reported.
-
DOSAGE AND ADMINISTRATION
The Ery 2% Pads should be rubbed over the affected area twice a day (morning and evening) after skin is thoroughly washed with warm water and soap and patted dry. Acne lesions on the face, neck, shoulders, chest, and back may be treated in this manner. Additional pledgets may be used, if needed. Each pledget should be used once and discarded. Wash hands after application. Close jar tightly after each use. Drying and peeling may be controlled by reducing the frequency of applications.
- HOW SUPPLIED
- STORAGE
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ERY
erythromycin swabProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63629-8648(NDC:45802-962) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ERYTHROMYCIN (UNII: 63937KV33D) (ERYTHROMYCIN - UNII:63937KV33D) ERYTHROMYCIN 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63629-8648-1 60 in 1 JAR 07/07/2008 1 0.8 mL in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA064126 07/07/2008 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 repack(63629-8648) , relabel(63629-8648)