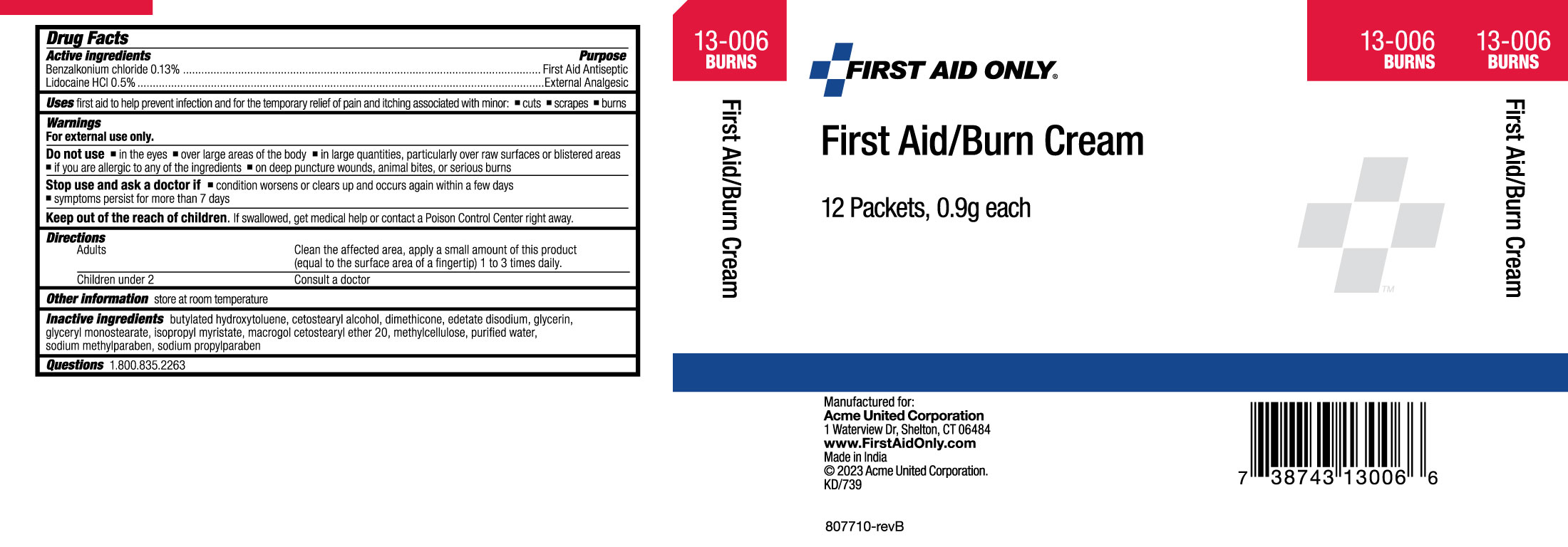
Label: FIRST AID ONLY FIRST AID/BURN- lidocaine hydrochloride, benzalkonium chloride cream
- NDC Code(s): 0924-5730-00, 0924-5730-02, 0924-5730-03
- Packager: Acme United Corporation
- This is a repackaged label.
- Source NDC Code(s): 61040-007
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
-
Warnings
For external use only.
Do not use
▪in the eyes ▪over large areas of the body ▪in large quantities, particularly over raw surfaces or blistered areas ▪if you are allergic to any of the ingredients ▪on deep puncture wounds, animal bites, or serious burns
- Directions
- Other Information
- Inactive Ingredients
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FIRST AID ONLY FIRST AID/BURN
lidocaine hydrochloride, benzalkonium chloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0924-5730(NDC:61040-007) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 5 mg in 1 g BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 g Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) WATER (UNII: 059QF0KO0R) EDETATE SODIUM (UNII: MP1J8420LU) METHYLPARABEN SODIUM (UNII: CR6K9C2NHK) PROPYLPARABEN SODIUM (UNII: 625NNB0G9N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0924-5730-00 0.9 g in 1 PACKET; Type 0: Not a Combination Product 10/16/2023 2 NDC:0924-5730-02 12 in 1 CARTON 10/16/2023 2 0.9 g in 1 PACKET; Type 0: Not a Combination Product 3 NDC:0924-5730-03 25 in 1 CARTON 10/16/2023 3 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 10/16/2023 Labeler - Acme United Corporation (001180207) Establishment Name Address ID/FEI Business Operations Acme United Corporation 045924339 relabel(0924-5730) , repack(0924-5730) Establishment Name Address ID/FEI Business Operations Acme United Corporation 080119599 repack(0924-5730) , relabel(0924-5730)