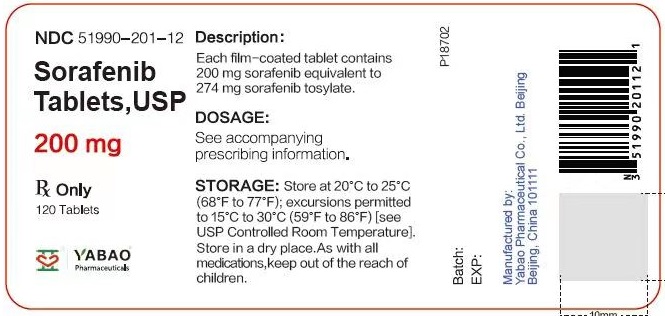
Label: SORAFENIB tablet, film coated
- NDC Code(s): 51990-201-06, 51990-201-12
- Packager: Yabao Pharmaceutical Co., Ltd. Beijing
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SORAFENIB TABLETS safely and effectively. See full prescribing information for SORAFENIB TABLETS. Sorafenib tablets, for oral ...
-
Table of ContentsTable of Contents
-
INDICATIONS & USAGE1.1 Hepatocellular Carcinoma - Sorafenib tablets are indicated for the treatment of patients with unresectable hepatocellular carcinoma (HCC). 1.3 Differentiated Thyroid Carcinoma - Sorafenib ...
-
DOSAGE & ADMINISTRATION2.1 Recommended Dosage - The recommended dosage of sorafenib tablets is 400 mg orallytwice daily without food (at least 1 hourbefore or 2 hours after a meal) until the patient is no longer ...
-
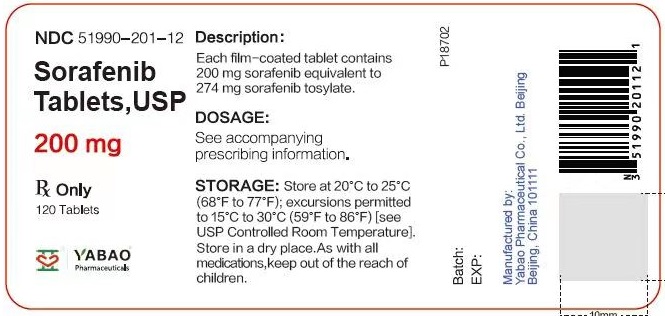
DOSAGE FORMS & STRENGTHS3 DOSAGE FORMS AND STRENGTHS - Sorafenib Tablets USP, 200 mg are round, pink, film-coated tablets, debossed with “YB” on one side and “201” on the other side.
-
CONTRAINDICATIONS4 CONTRAINDICATIONS - Sorafenib tablets are contraindicated in patients with known severe hypersensitivity to sorafenib or any other component of sorafenib tablets. Sorafenib tablets in ...
-
WARNINGS AND PRECAUTIONS5.1 Cardiovascular Events - In the SHARP (HCC) study, the incidence of cardiac ischemia/infarction was 2.7% in sorafenib tablets-treated patients compared with 1.3% in those receiving placebo, and ...
-
ADVERSE REACTIONS6 ADVERSE REACTIONS - The following clinically significant adverse reactions are discussed elsewhere in the labeling: Cardiovascular events [see Warnings and Precautions ( 5.1)] Hemorrhage ...
-
DRUG INTERACTIONS7.1 Effect of Other Drugs on Sorafenib Tablets - Strong CYP3A4 Inducers - The concomitant use of sorafenib tablets with rifampin, a strong CYP3A4 inducer decreased the mean AUC of sorafenib ...
-
USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], sorafenib tablets may cause fetal harm when administered to a ...
-
OVERDOSAGEThe adverse reactions observed at a dose of 800 mg twice daily (2 times the recommended dose) were primarily diarrhea and dermatologic. No information is available on symptoms of acute overdose in ...
-
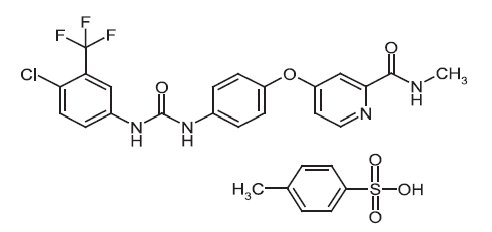
DESCRIPTIONSorafenib, a kinase inhibitor, is the tosylate salt of sorafenib. Sorafenib tosylate has the chemical name ...
-
CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sorafenib is a kinase inhibitor that decreases tumor cell proliferation in vitro. Sorafenib was shown to inhibit multiple intracellular (c-CRAF, BRAF and mutant BRAF ...
-
NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies have not been performed with sorafenib. Sorafenib was clastogenic when tested in an - in vitro mammalian cell ...
-
Patient Counseling InformationAdvise the patient to read FDA-approved patient labeling (Patient Information). Cardiovascular Events - Discuss with patients that cardiac ischemia and/or infarction and congestive heart ...
-
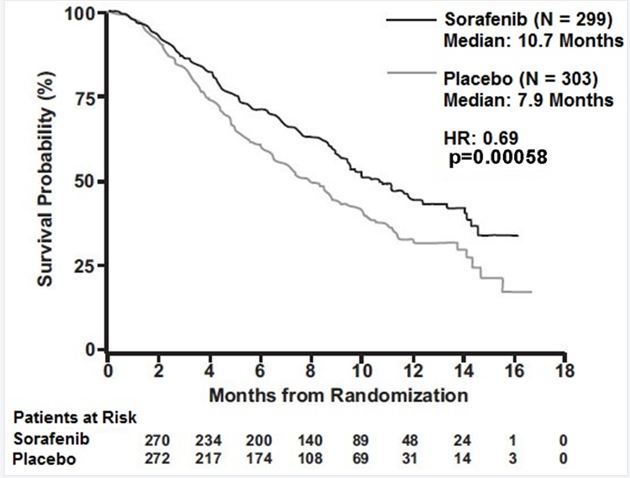
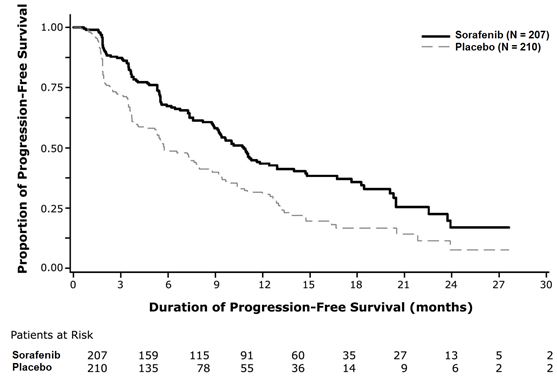
CLINICAL STUDIES14.1 Hepatocellular Carcinoma - The SHARP (HCC) study (NCT00105443) was an international, multicenter, randomized, double blind, placebo-controlled trial in patients with unresectable ...
-
HOW SUPPLIED/STORAGE AND HANDLINGSorafenib Tablets, USP are supplied as round, pink, film-coated tablets, debossed with “YB” on one side and “201” on the other side. Bottles of 120 tablets NDC 51990-201-12 - Bottles of 60 ...
-
PRINCIPAL DISPLAY PANELSorafenib tablets 200 mg Bottle Label - Rx Only - NDC 51990-201-06 - Sorafenib Tablets, USP - Each tablet contains - 200 mg - sorafenib - 60 Tablets - Sorafenib tablets 200 mg Bottle Label - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information