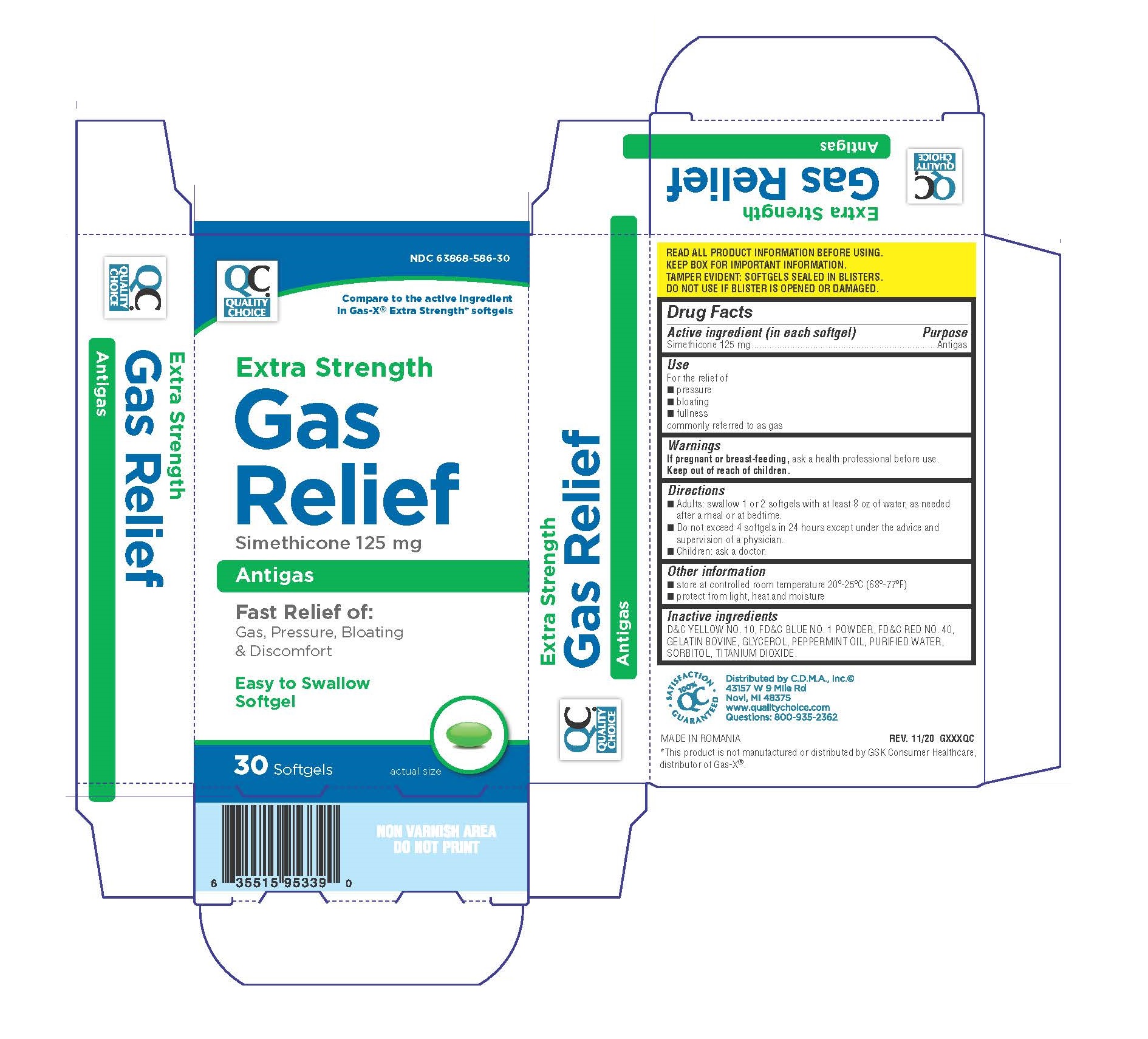
Label: QUALITY CHOICE GAS RELIEF EXTRA STRENGTH SOFTGELS- simethicone capsule, liquid filled
- NDC Code(s): 63868-586-30
- Packager: Chain Drug Marketing Association
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each softgel)
- Purpose
- Use
- Warnings If pregnant or breast-feeding, ask a health professional before use.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
QUALITY CHOICE GAS RELIEF EXTRA STRENGTH SOFTGELS
simethicone capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-586 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 125 mg Inactive Ingredients Ingredient Name Strength D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN TYPE B BOVINE (230 BLOOM) (UNII: WIL1404U79) GLYCERIN (UNII: PDC6A3C0OX) PEPPERMINT OIL (UNII: AV092KU4JH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SORBITOL (UNII: 506T60A25R) Product Characteristics Color GREEN Score no score Shape OVAL Size 10mm Flavor Imprint Code SCU Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-586-30 2 in 1 CARTON 12/02/2020 1 15 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M002 12/02/2020 Labeler - Chain Drug Marketing Association (011920774) Registrant - Reese Pharmaceutical Co (004172052) Establishment Name Address ID/FEI Business Operations Swiss Caps Romania 565466997 manufacture(63868-586)