Label: NGENLA- somatrogon-ghla injection, solution
- NDC Code(s): 0069-0505-01, 0069-0505-02, 0069-0520-01, 0069-0520-02
- Packager: Pfizer Laboratories Div Pfizer Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated April 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NGENLA safely and effectively. See full prescribing information for NGENLA.
NGENLA (somatrogon-ghla) injection, for subcutaneous use
Initial U.S. Approval: 2023INDICATIONS AND USAGE
NGENLA is a human growth hormone analog indicated for treatment of pediatric patients aged 3 years and older who have growth failure due to inadequate secretion of endogenous growth hormone (1).
DOSAGE AND ADMINISTRATION
- •
- NGENLA treatment should be supervised by a healthcare provider who is experienced in the diagnosis and management of pediatric patients with growth hormone deficiency (2.1).
- •
- Administer NGENLA by subcutaneous injection once weekly, on the same day each week, at any time of the day in the abdomen, thighs, buttocks, or upper arms with weekly rotation of injection site (2.1).
- •
- The recommended dosage is 0.66 mg/kg based on actual body weight administered once weekly (2.3).
- •
- Individualize dosage for each patient based on the growth response (2.3).
- •
- Patients switching from daily growth hormone may initiate treatment with once-weekly NGENLA on the day following their last daily injection (2.3).
- •
- If more than one injection is required to deliver a complete dose, each injection should be administered at a different injection site (2.3).
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- •
- Acute critical illness (4).
- •
- Hypersensitivity to somatrogon-ghla or excipients (4).
- •
- Closed epiphyses (4).
- •
- Active malignancy (4).
- •
- Active proliferative or severe non-proliferative diabetic retinopathy (4).
- •
- Prader-Willi syndrome who are severely obese or have severe respiratory impairment (4).
WARNINGS AND PRECAUTIONS
- •
- Severe Hypersensitivity: Severe hypersensitivity reactions may occur. In the event of an allergic reaction, seek prompt medical attention (5.2).
- •
- Increased Risk of Neoplasms: Monitor patients with preexisting tumors for progression or recurrence. Increased risk of a second neoplasm in childhood cancer survivors treated with somatropin – in particular meningiomas in patients treated with radiation to the head for their first neoplasm (5.3).
- •
- Glucose Intolerance and Diabetes Mellitus: NGENLA may decrease insulin sensitivity, particularly at higher doses. Monitor glucose levels periodically in all patients receiving NGENLA, especially in patients with existing diabetes mellitus or at risk for its development (5.4).
- •
- Intracranial Hypertension: Perform fundoscopic examinations prior to initiation of treatment with NGENLA and periodically thereafter. If preexisting papilledema is identified, evaluate the etiology and treat the underlying cause before initiating. If papilledema occurs with NGENLA, stop treatment (5.5).
- •
- Fluid Retention: May occur and may be dose dependent. Reduce dose as necessary (5.6).
- •
- Hypoadrenalism: Monitor patients for reduced serum cortisol levels and/or need for glucocorticoid dose increases in those with known hypoadrenalism (5.7).
- •
- Hypothyroidism: Monitor thyroid function periodically as hypothyroidism may become evident or worsen after initiation with NGENLA (5.8).
- •
- Slipped Capital Femoral Epiphysis: May develop. Evaluate patients with the onset of a limp or persistent hip or knee pain (5.9).
- •
- Progression of Preexisting Scoliosis: Monitor for development or progression of scoliosis (5.10).
- •
- Pancreatitis: Consider pancreatitis in patients with persistent severe abdominal pain (5.11).
- •
- Lipoatrophy: May occur if NGENLA is administered in the same location over a long period of time. Rotate injection sites (5.12).
ADVERSE REACTIONS
Adverse reactions reported in ≥5% of patients treated with NGENLA are: injection site reactions, nasopharyngitis, headache, pyrexia, anemia, cough, vomiting, hypothyroidism, abdominal pain, rash, and oropharyngeal pain (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
- •
- Replacement Glucocorticoid Treatment: Patients treated with glucocorticoid for hypoadrenalism may require an increase in their maintenance or stress dose following initiation of NGENLA (7).
- •
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Adjust glucocorticoid dosing in pediatric patients to avoid both hypoadrenalism and an inhibitory effect on growth (7).
- •
- Cytochrome P450-Metabolized Drugs: NGENLA may alter the clearance. Monitor carefully if used with NGENLA (7).
- •
- Oral Estrogen: Larger doses of NGENLA may be required (7).
- •
- Insulin and/or Other Antihyperglycemic Agents: Dose adjustment of insulin or antihyperglycemic agent may be required (5.4, 7).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing and Administration Information
2.2 Perform Fundoscopic Examination Prior to Initiation of NGENLA
2.3 Recommended Dosage and Monitoring for Pediatric Patients with GHD
2.4 Missed Dose
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Patients with Acute Critical Illness
5.2 Severe Hypersensitivity
5.3 Increased Risk of Neoplasms
5.4 Glucose Intolerance and Diabetes Mellitus
5.5 Intracranial Hypertension
5.6 Fluid Retention
5.7 Hypoadrenalism
5.8 Hypothyroidism
5.9 Slipped Capital Femoral Epiphysis
5.10 Progression of Preexisting Scoliosis
5.11 Pancreatitis
5.12 Lipoatrophy
5.13 Sudden Death in Pediatric Patients with Prader-Willi Syndrome
5.14 Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Treatment-Naïve Pediatric Patients with Growth Hormone Deficiency
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing and Administration Information
- •
- NGENLA treatment should be supervised by a healthcare provider who is experienced in the diagnosis and management of pediatric patients aged 3 years and older with growth failure due to growth hormone deficiency (GHD) [see Indications and Usage (1)].
- •
- Refer patient to the Instructions for Use for complete administration instructions.
- •
- Administer NGENLA by subcutaneous injection, once weekly, on the same day each week, at any time of the day in the abdomen, thighs, buttocks, or upper arms. Rotate the injection site weekly.
- •
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If flakes, particles or discoloration are observed, do not use the pen. Do not shake; shaking can damage the product.
- •
- Prefilled pens deliver somatrogon-ghla in 0.2 mg or 0.5 mg increments.
2.2 Perform Fundoscopic Examination Prior to Initiation of NGENLA
- •
- Perform fundoscopic examination before initiating treatment with NGENLA to exclude preexisting papilledema. If papilledema is identified, evaluate the etiology and treat the underlying cause before initiating treatment with NGENLA [see Warnings and Precautions (5.4)].
2.3 Recommended Dosage and Monitoring for Pediatric Patients with GHD
- •
- Recommended dosage of NGENLA is 0.66 mg/kg based on actual body weight administered once weekly by subcutaneous (SC) injection.
- •
- Individualize dosage for each patient based on the growth response.
- •
- The day of weekly administration can be changed if necessary as long as the time between 2 doses is at least 3 days. After selecting a new dosing day, the once weekly dosing should be continued.
- •
- When switching from daily growth hormone, the once-weekly NGENLA may be initiated on the day following their last daily injection.
- •
- If more than one injection is required to deliver a complete dose, each injection should be administered at a different injection site.
-
3 DOSAGE FORMS AND STRENGTHS
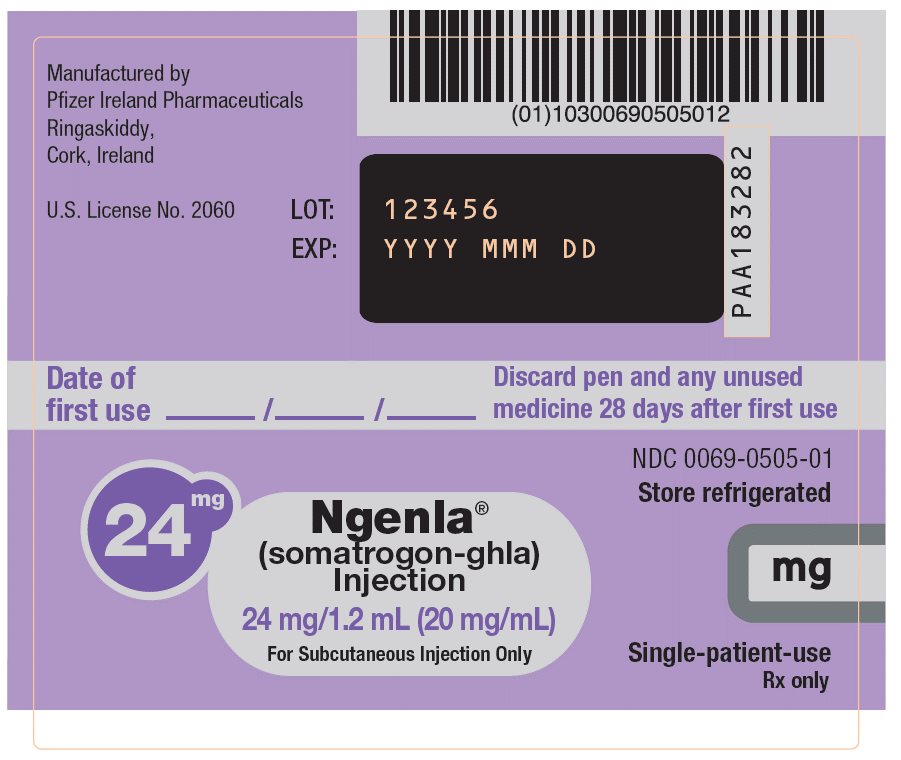
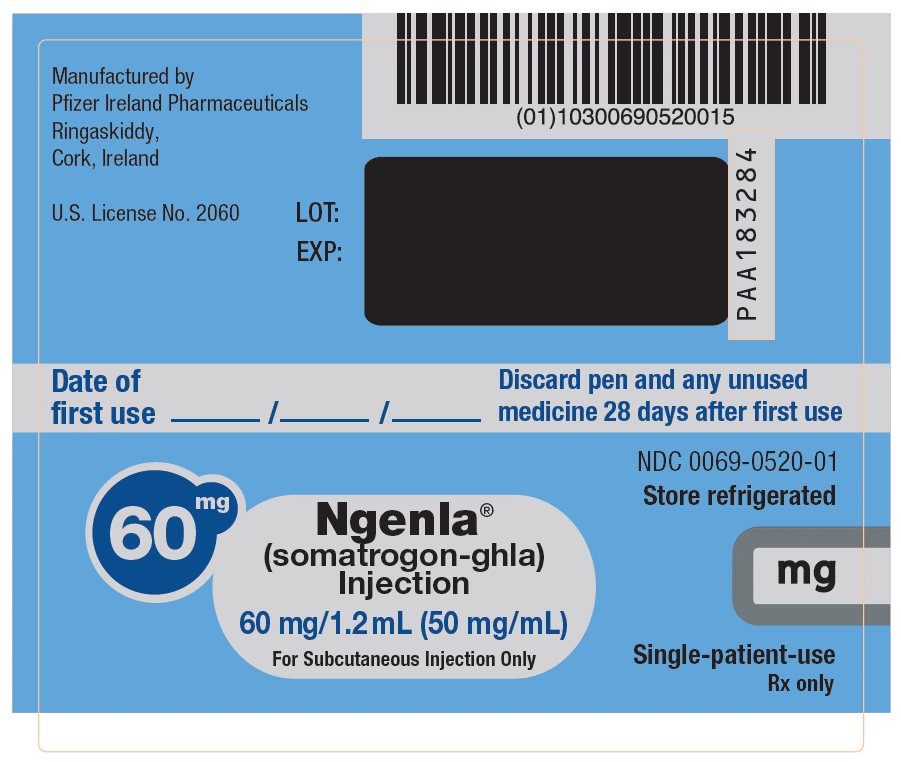
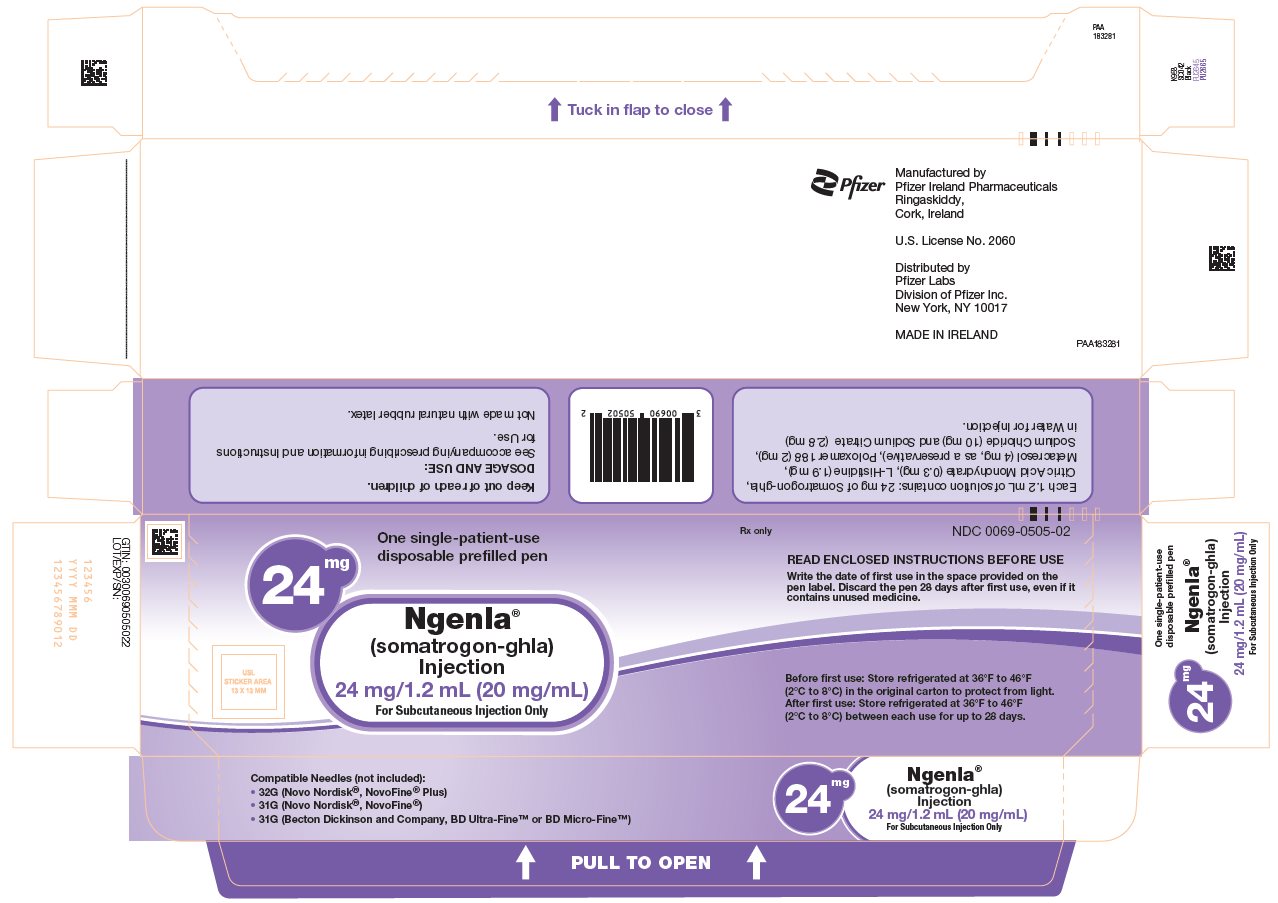
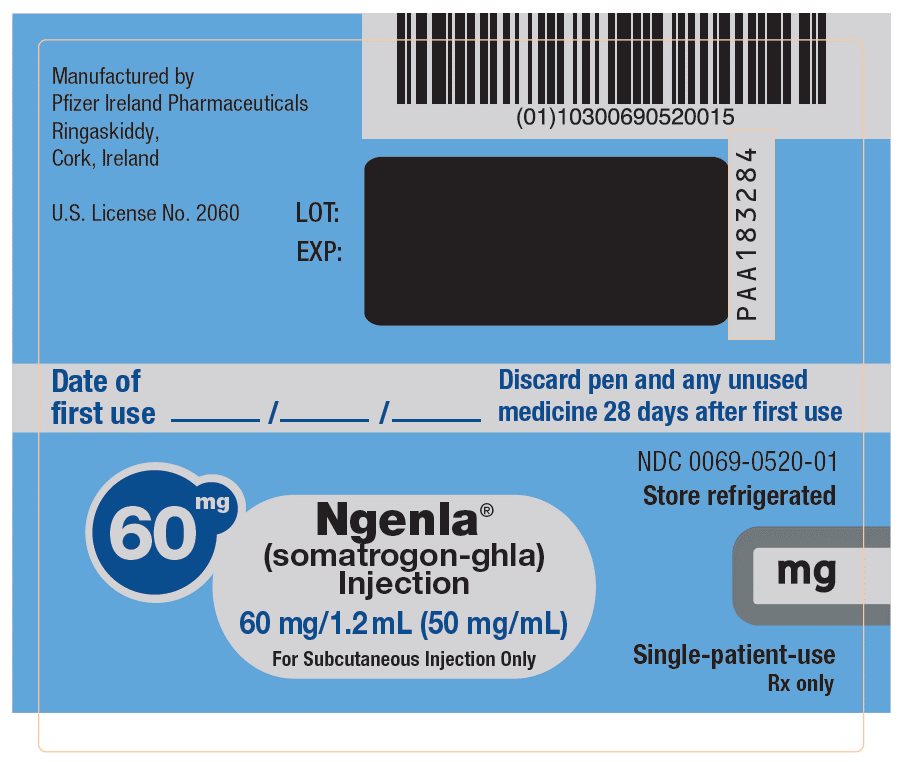
NGENLA (somatrogon-ghla) is a clear and colorless to slightly light yellow solution available as:
- •
- Injection: 24 mg/1.2 mL (20 mg/mL) in a single-patient-use, disposable prefilled pen that delivers a dose in 0.2 mg increments.
- •
- Injection: 60 mg/1.2 mL (50 mg/mL) in a single-patient-use, disposable prefilled pen that delivers a dose in 0.5 mg increments.
-
4 CONTRAINDICATIONS
- •
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with somatropin [see Warnings and Precautions (5.1)].
- •
- Hypersensitivity to somatrogon-ghla or any of the excipients in NGENLA [see Warnings and Precautions (5.2)].
- •
- Closed epiphyses.
- •
- Active malignancy due to the risk of malignancy progression [see Warnings and Precautions (5.3)].
- •
- Active proliferative or severe non-proliferative diabetic retinopathy [see Warnings and Precautions (5.4)].
- •
- Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea or have severe respiratory impairment due to the risk of sudden death [see Warnings and Precautions (5.13)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Patients with Acute Critical Illness
Increased mortality in patients with acute critical illness due to complications following open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure has been reported with somatropin [see Contraindications (4)]. The safety of continuing NGENLA treatment for the approved indication in patients who concurrently develop these illnesses has not been established.
5.2 Severe Hypersensitivity
Severe systemic hypersensitivity reactions including anaphylaxis and angioedema have been reported with somatropin. Inform patients and/or caregivers that such reactions are possible and that prompt medical attention should be sought if an allergic reaction occurs. NGENLA is contraindicated in patients with known hypersensitivity to somatrogon-ghla or any excipients in NGENLA [see Contraindications (4)].
5.3 Increased Risk of Neoplasms
Active Malignancy
There is an increased risk of malignancy progression with somatropin treatment in patients with active malignancy [see Contraindications (4)]. Any preexisting malignancy should be inactive, and its treatment should be completed prior to instituting therapy with NGENLA. Discontinue NGENLA if there is evidence of recurrent malignancy.
Risk of Second Neoplasm in Pediatric Patients
In childhood cancer survivors, who were treated with radiation to the brain/head for their first neoplasm and who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm has been reported. Intracranial tumors, in particular meningiomas, were the most common of these second neoplasms. Monitor all patients with a history of GHD secondary to an intracranial neoplasm while on NGENLA therapy for progression or recurrence of the tumor.
New Malignancy During Treatment
Because children with certain rare genetic causes of short stature have an increased risk of developing malignancies, thoroughly consider the risks and benefits of starting NGENLA in these patients. If treatment with NGENLA is initiated, carefully monitor these patients for development of neoplasms.
Monitor patients on NGENLA therapy carefully for increased growth or potential malignant changes of preexisting nevi. Advise patients and/or caregivers to report marked changes in behavior, onset of headaches, vision disturbances and/or changes in skin pigmentation or changes in the appearance of preexisting nevi.
5.4 Glucose Intolerance and Diabetes Mellitus
Treatment with growth hormone may decrease insulin sensitivity, particularly at higher doses. New onset type 2 diabetes mellitus has been reported in patients receiving growth hormone. Patients with undiagnosed pre-diabetes and diabetes mellitus may experience worsened glycemic control and become symptomatic. Monitor glucose levels periodically in all patients receiving NGENLA, especially in those with risk factors for diabetes mellitus, such as obesity, Turner syndrome, or a family history of diabetes mellitus. Patients with preexisting type 1 or type 2 diabetes mellitus or pre-diabetes should be monitored closely. The doses of antidiabetic agents may require adjustment when NGENLA is initiated.
5.5 Intracranial Hypertension
Intracranial hypertension (IH) with papilledema, visual changes, headache, nausea, and/or vomiting has been reported in patients treated with somatropin. Symptoms usually occurred within the first eight (8) weeks after the initiation of somatropin therapy. In all reported cases, IH-associated signs and symptoms rapidly resolved after cessation of therapy or a reduction of somatropin dose.
Perform fundoscopic examination before initiating treatment with NGENLA to exclude preexisting papilledema and periodically thereafter. If papilledema is identified prior to initiation, evaluate the etiology and treat the underlying cause before initiating NGENLA. NGENLA should be temporarily discontinued in patients with clinical or fundoscopic evidence of IH. If IH is confirmed, restart treatment with NGENLA at a lower dose after IH-associated signs and symptoms have resolved.
5.6 Fluid Retention
Fluid retention during NGENLA therapy may occur. Clinical manifestations of fluid retention (e.g. edema and nerve compression syndromes including carpal tunnel syndrome/paresthesia) are usually transient and dose dependent.
5.7 Hypoadrenalism
Patients receiving growth hormone therapy who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of NGENLA treatment. Monitor patients for reduced serum cortisol levels and/or need for glucocorticoid dose increases in those with known hypoadrenalism [see Drug Interactions (7)].
5.8 Hypothyroidism
Undiagnosed/untreated hypothyroidism may prevent an optimal response to NGENLA therapy. In patients with GH deficiency, central (secondary) hypothyroidism may first become evident or worsen during treatment with growth hormone therapy. Therefore, patients should have periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or appropriately adjusted when indicated.
5.9 Slipped Capital Femoral Epiphysis
Slipped capital femoral epiphysis may occur more frequently in patients undergoing rapid growth. Evaluate pediatric patients with the onset of a limp or complaints of persistent hip or knee pain.
5.10 Progression of Preexisting Scoliosis
NGENLA increases growth rate, and progression of preexisting scoliosis can occur in patients who experience rapid growth. Growth hormone treatment has not been shown to increase the occurrence of scoliosis. Monitor patients with a history of scoliosis for disease progression.
5.11 Pancreatitis
Cases of pancreatitis have been reported in patients receiving somatropin. The risk may be greater in pediatric patients compared with adults. Consider pancreatitis in patients who develop persistent severe abdominal pain.
5.12 Lipoatrophy
When NGENLA is administered subcutaneously at the same site over a long period of time, lipoatrophy may result. Rotate injection sites when administering NGENLA to reduce this risk [see Dosage and Administration (2.1)].
5.13 Sudden Death in Pediatric Patients with Prader-Willi Syndrome
There have been reports of sudden death after initiating therapy with somatropin in pediatric patients with Prader-Willi syndrome who had one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Male patients with one or more of these factors may be at greater risk than females. NGENLA is not indicated for the treatment of pediatric patients who have growth failure due to genetically confirmed Prader-Willi syndrome.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- Increased mortality in patients with acute critical illness [see Warnings and Precautions (5.1)]
- •
- Severe hypersensitivity [see Warnings and Precautions (5.2)]
- •
- Increased risk of neoplasm [see Warnings and Precautions (5.3)]
- •
- Glucose intolerance and diabetes mellitus [see Warnings and Precautions (5.4)]
- •
- Intracranial hypertension [see Warnings and Precautions (5.5)]
- •
- Fluid retention [see Warnings and Precautions (5.6)]
- •
- Hypoadrenalism [see Warnings and Precautions (5.7)]
- •
- Hypothyroidism [see Warnings and Precautions (5.8)]
- •
- Slipped capital femoral epiphysis [see Warnings and Precautions (5.9)]
- •
- Progression of preexisting scoliosis [see Warnings and Precautions (5.10)]
- •
- Pancreatitis [see Warnings and Precautions (5.11)]
- •
- Lipoatrophy [see Warnings and Precautions (5.12)]
- •
- Sudden death in pediatric patients with Prader-Willi syndrome [see Warnings and Precautions (5.13)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Safety data are derived from a safety and efficacy study in pediatric patients with GHD [see Clinical Studies (14.1)]. The data from the 12-month main study period reflect exposure of 109 patients to NGENLA administered once weekly (0.66 mg/kg/wk) and 115 patients to somatropin administered once daily (0.034 mg/kg/day).
The mean age across the treatment groups, was 7.7 years (min 3.01, max 11.96); 40.2% of patients were >3 years to ≤7 years, 59.8% were >7 years, 71.9% of patients were male, and 28.1% were female. In this study, 74.6% of patients were White, 20.1% were Asian, 0.9% were Black or African American, 0.5% were American Indian or Alaska Native, 0.5% were Native Hawaiian or Other Pacific Islander, and for 3.6% race information was missing; 10.7% of patients identified as Hispanic or Latino. Baseline disease characteristics were balanced across treatment groups.
Table 1 shows the adverse reactions that occurred in ≥5% of patients treated with NGENLA or daily somatropin during the 12-month main study period. Reporting of injection site reactions was solicited through the use of a patient diary after each weekly injection for patients administered NGENLA and once weekly for patients administered daily injections of somatropin.
Table 1 Adverse Reactions Occurring in ≥5% of NGENLA- or Somatropin-Treated Pediatric Patients (52 Weeks of Treatment) Adverse reactions that are medically related were grouped to a single preferred term. - *
- Injection site reactions included: injection site pain (39% somatrogon-ghla vs 25% daily somatropin), injection site swelling/induration/hypertrophy/inflammation (10% somatrogon-ghla vs 1% daily somatropin), injection site erythema (8% somatrogon-ghla vs none daily somatropin), injection site pruritus (5% somatrogon-ghla vs none daily somatropin), injection site hemorrhage (5% somatrogon-ghla vs none daily somatropin).
- †
- Nasopharyngitis included: rhinitis, pharyngitis, rhinitis allergic, pharyngitis streptococcal, viral pharyngitis, nasopharyngitis.
Adverse Drug Reactions
Daily Somatropin
(N=115)
n (%)
NGENLA
(N=109)
n (%)
Injection site reactions*
29 (25.2)
46 (42.2)
Nasopharyngitis†
33 (28.7)
36 (33)
Headache
25 (21.7)
18 (16.5)
Pyrexia
17 (14.8)
18 (16.5)
Anemia
10 (8.7)
10 (9.2)
Cough
9 (7.8)
9 (8.3)
Vomiting
9 (7.8)
8 (7.3)
Hypothyroidism
3 (2.6)
7 (6.4)
Abdominal pain
8 (7.0)
7 (6.4)
Rash
7 (6.1)
6 (5.5)
Oropharyngeal pain
4 (3.5)
6 (5.5)
Arthralgia
8 (7.0)
5 (4.6)
Otitis media
10 (8.7)
5 (4.6)
Tonsillitis
6 (5.2)
5 (4.6)
Bronchitis
9 (7.8)
3 (2.8)
Laboratory Tests
More NGENLA-treated patients shifted from normal eosinophil levels at baseline to elevated eosinophil levels at the end of the 12-month study compared to the daily somatropin group (29% vs 12%).
-
7 DRUG INTERACTIONS
Table 2 includes a list of drugs with clinically significant drug interactions when administered concomitantly with NGENLA and instructions for preventing or managing them.
Table 2 Clinically Significant Drug Interactions with NGENLA Replacement Glucocorticoid Treatment
Clinical Impact:
Microsomal enzyme 11β-hydroxysteroid dehydrogenase type 1 (11βHSD-1) is required for conversion of cortisone to its active metabolite, cortisol, in hepatic and adipose tissue. Growth hormone inhibits 11βHSD-1. Consequently, individuals with untreated GH deficiency have relative increases in 11βHSD-1 and serum cortisol. Initiation of NGENLA may result in inhibition of 11βHSD-1 and reduced serum cortisol concentrations.
Intervention:
Patients treated with glucocorticoid replacement for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of NGENLA [see Warnings and Precautions (5.7)].
Examples:
Cortisone acetate and prednisone may be affected more than others because conversion of these drugs to their biologically active metabolites is dependent on the activity of 11βHSD-1.
Supraphysiologic Glucocorticoid Treatment
Clinical Impact:
Supraphysiologic glucocorticoid treatment may attenuate the growth-promoting effects of NGENLA in pediatric patients.
Intervention:
Carefully adjust glucocorticoid replacement dosing in pediatric patients receiving glucocorticoid treatments to avoid hypoadrenalism and an inhibitory effect on growth.
Cytochrome P450-Metabolized Drugs
Clinical Impact:
Limited published data indicate that growth hormone treatment increases cytochrome P450 (CYP450)-mediated antipyrine clearance. NGENLA may alter the clearance of compounds known to be metabolized by CYP450 liver enzymes.
Intervention:
Careful monitoring is advisable when NGENLA is administered in combination with drugs metabolized by CYP450 liver enzymes.
Oral Estrogen
Clinical Impact:
Oral estrogens may reduce the serum IGF-1 response to NGENLA.
Intervention:
Patients receiving oral estrogen replacement may require higher NGENLA dosages.
Insulin and/or Other Antihyperglycemic Agents
Clinical Impact:
Treatment with NGENLA may decrease insulin sensitivity, particularly at higher doses.
Intervention:
Patients with diabetes mellitus may require adjustment of their doses of insulin and/or other antihyperglycemic agents [see Warnings and Precautions (5.4)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on NGENLA use in pregnant women to evaluate for a drug associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In reproduction studies with pregnant rats, there was no evidence of embryo-fetal toxicity following administration of somatrogon-ghla subcutaneously during organogenesis at doses up to 45 times the maximum recommended human dose based on exposure (see Data).
The background risk of major birth defects and miscarriage in the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Animal Data
In an embryo-fetal development toxicity study in rats, no adverse maternal or embryo-fetal effects were observed when somatrogon-ghla was administered via subcutaneous injection every 2 days from gestation day (GD) 6 to 18 at doses up to 30 mg/kg (45 times the maximum recommended human dose based on Cav exposure).
In a pre- and postnatal development study in rats, somatrogon-ghla was administered via subcutaneous injection to pregnant rats every 2 days from GD 6 to lactation day 20 at doses up to 30 mg/kg. There was no evidence of maternal toxicity and no adverse effects on the first generation (F1) offspring. Somatrogon-ghla elicited an increase in F1 mean body weights in both sexes and increased the mean copulatory interval in F1 females at the highest dose (30 mg/kg), consistent with a longer estrous cycle length. However, there were no effects on mating indices in F1 females.
8.2 Lactation
Risk Summary
There are no data on the presence of somatrogon-ghla in human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for NGENLA and any potential adverse effects on the breastfed infant from NGENLA or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Although somatrogon-ghla did not interfere with hCG pregnancy testing in a limited number of commercial tests, interference with hCG blood and urine pregnancy testing in patients receiving somatrogon-ghla may be possible, leading to either false positive or false negative results. Alternative methods (i.e., not reliant on hCG) are recommended to determine pregnancy.
8.4 Pediatric Use
The safety and effectiveness of NGENLA have been established for the treatment of growth failure due to inadequate secretion of endogenous growth hormone (GH) in pediatric patients aged 3 years and older [see Clinical Studies (14.1)]. The use of NGENLA for this indication is supported by evidence from a 52‑week, multi-center, randomized, open-label, active-controlled, parallel-group phase 3 study in 224 treatment-naïve, prepubertal pediatric subjects with growth hormone deficiency.
Risks in pediatric patients associated with growth hormone use include:
- •
- Increased risk of second neoplasm in pediatric cancer survivors treated with radiation to the brain and/or head [see Warnings and Precautions (5.3)]
- •
- Slipped capital femoral epiphysis [see Warnings and Precautions (5.9)]
- •
- Progression of preexisting scoliosis [see Warnings and Precautions (5.10)]
- •
- Pancreatitis [see Warnings and Precautions (5.11)]
- •
- Sudden death in pediatric patients with Prader-Willi Syndrome. NGENLA is not indicated for the treatment of pediatric patients with growth failure secondary to genetically confirmed Prader‑Willi syndrome. [see Warnings and Precautions (5.13)]
- 10 OVERDOSAGE
-
11 DESCRIPTION
Somatrogon-ghla, a human growth hormone analog, is a fusion protein produced in Chinese Hamster Ovary (CHO) cells by recombinant DNA technology. It is comprised of the amino acid sequence of human growth hormone (hGH) with one copy of the C-terminal peptide (CTP) from the beta chain of human chorionic gonadotropin (hCG) at the N-terminus and 2 copies of CTP (in tandem) at the C-terminus. Somatrogon-ghla has an approximate molecular weight of 40 KDa.
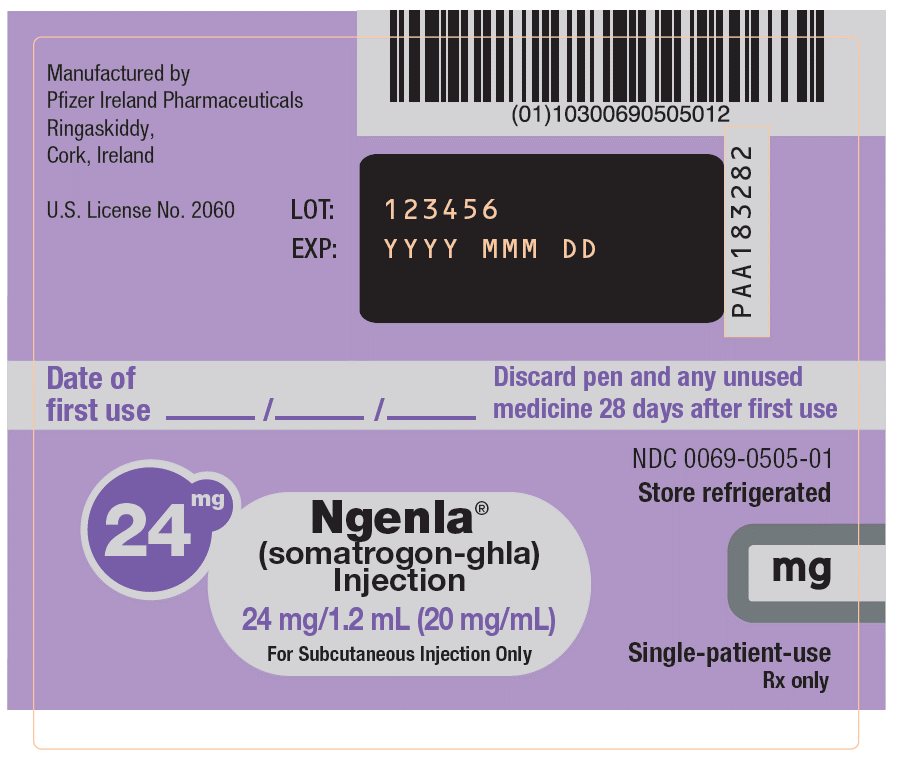
NGENLA (somatrogon-ghla) injection is a sterile, clear and colorless to slightly light yellow solution for subcutaneous use supplied in a 24 mg/1.2 mL (20 mg/mL) or 60 mg/1.2 mL (50 mg/mL) single-patient-use prefilled pen.
Each 1.2 mL of solution contains either 24 mg or 60 mg of somatrogon-ghla, and the inactive ingredients citric acid monohydrate (0.3 mg), histidine (1.9 mg), metacresol (4 mg, as a preservative), poloxamer 188 (2 mg), sodium chloride (10 mg) and sodium citrate (2.8 mg) in water for injection. NGENLA has a pH of approximately 6.6.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Somatrogon-ghla binds to the GH receptor and initiates a signal transduction cascade culminating in changes in growth and metabolism. Somatrogon-ghla binding leads to activation of the STAT5b signaling pathway and increases the serum concentration of Insulin-like Growth Factor (IGF-1). GH and IGF-1 stimulate metabolic changes, linear growth, and enhance growth velocity in pediatric patients with GHD.
12.2 Pharmacodynamics
Following single dose administration of somatrogon, dose-dependent increases in IGF-1 response were observed.
Following multiple dosing, IGF-1 SDS levels were in the normal range for pediatric patients with GHD, similar to daily somatropin. IGF-1 levels peak approximately 2 days (48 hours) post-dose, with the average weekly IGF-1 occurring approximately 4 days post-dose.
12.3 Pharmacokinetics
Somatrogon-ghla pharmacokinetics (PK) was assessed using a population PK approach for NGENLA in 151 pediatric patients (aged 3 to 15.5 years) with GHD.
Absorption
Following subcutaneous injection, serum concentrations increased slowly, peaking 6 to 25 hours with a median of 11 hours after dosing.
In pediatric patients with GHD, somatrogon-ghla exposure increases in a dose-proportional manner for doses of 0.25 mg/kg/wk, 0.48 mg/kg/wk, and 0.66 mg/kg/wk. There is no accumulation of somatrogon-ghla after once weekly administration. In pediatric patients with GHD, the mean population PK estimated steady-state peak concentrations (mean ± SD) following 0.66 mg/kg/wk was 495 ± 90 ng/mL.
Distribution
In pediatric patients with GHD, the mean population PK estimated apparent central volume of distribution was 0.342 L/kg and apparent peripheral volume of distribution was 0.671 L/kg.
Elimination
In pediatric patients with GHD, the mean population PK estimated apparent clearance was 0.0398 L/h/kg. The mean population PK estimated effective half-life was 37.7 hours, which allows for weekly dosing. Somatrogon-ghla will be present in the circulation for about 8 days after the last dose.
Specific Populations
Based on population PK analyses, age, sex, race, and ethnicity do not have a clinically meaningful effect on the pharmacokinetics of somatrogon-ghla in pediatric patients with GHD. The exposure of somatrogon-ghla decreases with an increase in body weight. However, the somatrogon-ghla dosing regimen of 0.66 mg/kg/wk provides adequate systemic exposure over the body weight range of 10 to 54 kg evaluated in the clinical studies.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of somatrogon or other growth hormone products.
During the 12-month main period of study NCT 02968004, 84/109 (77.1%) of somatrogon-ghla‑treated patients tested positive for anti-drug antibodies, with most showing specificity to human growth hormone. The anti-drug antibodies persisted in most of the subjects during the study. Neutralizing antibodies developed in 8/217 (3.7%) of somatrogon-ghla-treated patients during the study for up to 42 months of exposure to somatrogon-ghla. The neutralizing antibodies were transient in all subjects. Anti-drug antibodies, including neutralizing-antibodies, did not appear to have a clinically significant impact on the safety or effectiveness of NGENLA during the 12‑month randomized treatment period. Additionally, no apparent effect of anti-drug antibodies on growth was observed for additional 30 months of exposure to somatrogon-ghla in the uncontrolled extension period of study NCT 02968004.
Anti-Drug Antibody Effects on Pharmacokinetics
The population pharmacokinetic analysis of data from study NCT 02968004 showed that patients who tested positive for anti-drug antibodies had an approximately 26% decrease in apparent clearance. These anti-drug antibody-associated pharmacokinetic changes are not considered to be clinically significant.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Impairment of Fertility
The potential for somatrogon-ghla to affect fertility and early embryonic development was evaluated in male and female rats administered subcutaneously before cohabitation, through mating to implantation. Somatrogon‑ghla elicited an increase in estrous cycle length, copulatory interval, and number of corpora lutea at exposures ≥22-fold the MRHD, but there was no impact on female fertility, mating indices, number of viable embryos or early embryonic development, or on male fertility up to 30 mg/kg every two days (45-fold the MRHD based on exposure).
-
14 CLINICAL STUDIES
14.1 Treatment-Naïve Pediatric Patients with Growth Hormone Deficiency
A multi-center, randomized, open-label, active-controlled, parallel-group phase 3 study (NCT 02968004) was conducted in 224 treatment-naïve, prepubertal pediatric subjects with growth hormone deficiency (GHD). The primary efficacy endpoint was annualized height velocity at Week 52.
One hundred nine (109) subjects received 0.66 mg/kg/week NGENLA, and 115 subjects received 0.034 mg/kg/day daily somatropin. The subjects age ranged from 3 to 12 years, with a mean of 7.7 years. One hundred sixty-one (71.9%) subjects were male and 63 (28.1%) were female. One hundred sixty-seven (74.6%) subjects were White, 45 (20.1%) subjects were Asian, 2 (0.9%) subjects were Black or African-American, 1 (0.5%) subject was American Indian or Alaska Native, 1 (0.5%) subject was Native Hawaiian or Other Pacific Islander, and for 8 (3.6%) subjects race information was missing; 24 (10.7%) subjects identified as Hispanic or Latino. The subjects had a mean baseline height standard deviation score (SDS) of -2.9.
Treatment with once-weekly NGENLA for 52 weeks resulted in an annualized height velocity of 10.1 cm/year. Patients treated with daily somatropin achieved an annualized height velocity of 9.8 cm/year after 52 weeks of treatment. Refer to Table 3.
Table 3. Annualized Height Velocity at Week 52 in Pediatric Patients with GHD Abbreviations: CI=confidence interval; LSM=least square mean; N=number of patients randomized and treated The estimates of LSM are from analysis of covariance model with treatment, age group, gender, peak growth hormone levels, and region as fixed factors and baseline height SDS as a covariate. Missing data is imputed by multiple imputation using SAS PROC MI with MNAR/FCS Method. Treatment Parameter
Treatment Group
LSM Treatment Difference
(95% CI) (NGENLA minus Daily Somatropin)NGENLA
(N=109)Daily Somatropin
(N=115)LSM Estimate
LSM Estimate
Annualized Height Velocity (cm/yr)
10.1
9.8
0.3 (-0.2, 0.9)
The mean height SDS at Week 52 was -1.94 in NGENLA arm and -1.99 in the daily somatropin arm. The mean increase in height SDS from baseline at Week 52 was 0.92 in NGENLA arm and 0.87 in the daily somatropin arm, respectively.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
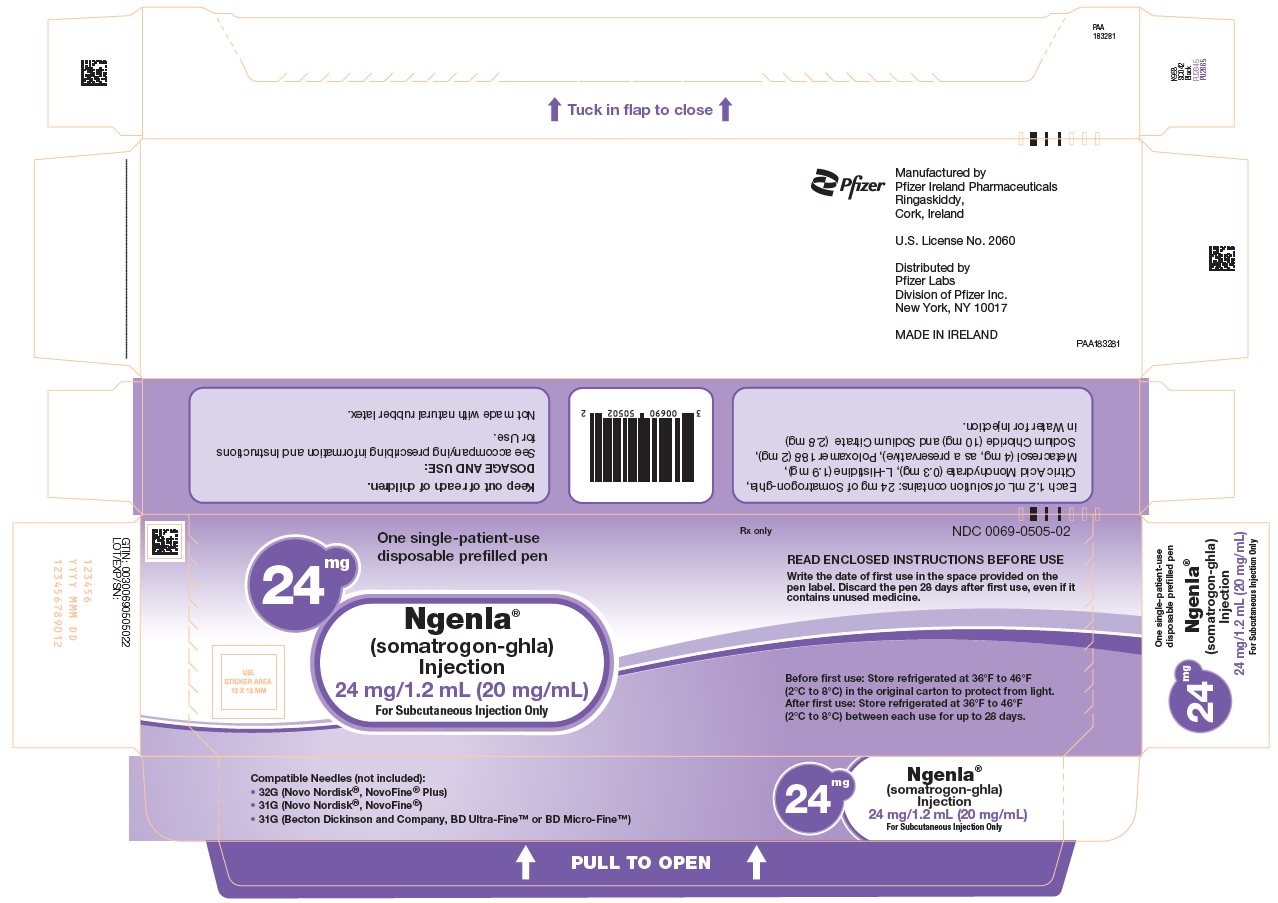
NGENLA (somatrogon-ghla) injection is a clear and colorless to slightly light yellow solution containing a preservative and supplied as one single-patient-use disposable prefilled pen per carton available in the following packages:
24 mg/1.2 mL Prefilled Pen
NDC: 0069-0505-0260 mg/1.2 mL Prefilled Pen
NDC: 0069-0520-02Somatrogon-ghla solution concentration
20 mg/mL
50 mg/mL
Color scheme
Lilac pen cap, injection button and label
Blue pen cap, injection button and label
Dose increments
0.2 mg/0.01 mL
0.5 mg/0.01 mL
Maximum dose
12 mg (0.6 mL)
30 mg (0.6 mL)
Not made with natural rubber latex.
Sterile needles are required for administration but not included. Consult the Instructions for Use for needles that can be used.
Storage and Handling
Before first use: Store refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light.
After first use: Store the pen refrigerated at 36°F to 46°F (2°C to 8°C) between each use, for up to 28 days.
Do not freeze or shake. Do not expose to heat. Do not use if it has been frozen. Store away from direct sunlight.
Always remove and safely discard the needle after each injection and store the NGENLA prefilled pen without an injection needle attached. Always use a new needle for each injection. Replace the cap on your prefilled pen when it is not in use. Write the date of first use in the space provided on the pen label. The prefilled pen should not be used more than 28 days after first use.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- •
-
Hypersensitivity Reactions
Advise patients and caregivers that serious systemic hypersensitivity reactions (anaphylaxis and angioedema) are possible and that prompt medical attention should be sought if an allergic reaction occurs [see Warnings and Precautions (5.2)].
- •
-
Neoplasm
Advise childhood cancer survivors and caregivers that individuals treated with radiation to the head are at increased risk of secondary neoplasms and, as a precaution, need to be monitored for recurrence. Advise patients to report marked changes in skin pigmentation or changes in the appearance of preexisting nevi [see Warnings and Precautions (5.3)].
- •
-
Glucose Intolerance/Diabetes Mellitus
Advise patients and caregivers that new onset of insulin resistance and hyperglycemia may occur and monitoring of blood glucose during treatment with NGENLA in patients with glucose intolerance or who have risk factors for diabetes, may be needed [see Warnings and Precautions (5.4)].
- •
-
Intracranial Hypertension
Advise patients and caregivers to report to their healthcare provider any visual changes, headache, and nausea and/or vomiting [see Warnings and Precautions (5.5)].
- •
-
Fluid Retention
Advise patients and caregivers that fluid retention during NGENLA therapy may occur. Inform patients of the clinical manifestations of fluid retention (e.g. edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paresthesia) and to report to their healthcare provider if any of these signs or symptoms occur during treatment with NGENLA.
- •
-
Hypoadrenalism
Advise patients and caregivers who have or who are at risk for corticotropin deficiency that hypoadrenalism may develop and to report to their healthcare provider if extreme fatigue, dizziness, weakness, vomiting, dehydration or weight loss is experienced during treatment with NGENLA [see Warnings and Precautions (5.7)].
- •
-
Hypothyroidism
Advise patients and caregivers that undiagnosed/untreated hypothyroidism may prevent an optimal response to NGENLA. Advise patients and caregivers they may require periodic thyroid function tests during treatment with NGENLA [see Warnings and Precautions (5.8)].
- •
-
Pancreatitis
Advise patients and caregivers that pancreatitis may develop and to report to their healthcare provider any new onset persistent severe abdominal pain.
- •
-
Lipoatrophy
Advise patients and caregivers that lipoatrophy may occur if NGENLA is administered subcutaneously at the same site over a long period of time. Advise patients to rotate injection sites when administering NGENLA to reduce this risk.
-
SPL UNCLASSIFIED SECTION
Manufactured by:
Pfizer Ireland Pharmaceuticals
Ringaskiddy,
Cork,
IrelandUS License No: 2060
This product’s labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
LAB-1433-1.0
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
NGENLA® (en’ JEN-lah)(somatrogon-ghla)
injection, for subcutaneous use
What is NGENLA?
NGENLA is a prescription medicine that contains a form of human growth hormone, like the growth hormone made by the human body. NGENLA is given by injection under the skin (subcutaneous) in children who are not growing because of low or no growth hormone levels.Do not use NGENLA if:
- •
- your child has a critical illness caused by certain types of heart or stomach surgery, trauma or breathing (respiratory) problems.
- •
- your child is allergic to somatrogon-ghla or any of the ingredients in NGENLA. See the end of this leaflet for a complete list of ingredients in NGENLA.
- •
- your child has closed bone growth plates (epiphyses).
- •
- your child has cancer or other tumors.
- •
- your child’s healthcare provider tells you that your child has certain types of eye problems caused by diabetes (diabetic retinopathy).
- •
- your child has Prader-Willi syndrome, is severely obese, or has breathing problems including sleep apnea (briefly stop breathing during sleep).
Before using NGENLA, tell your child’s healthcare provider about all of your child’s medical conditions, including if your child:
- •
- has had heart or stomach surgery, trauma or serious breathing (respiratory) problems.
- •
- has had a history of problems breathing while they slept (sleep apnea).
- •
- has or has had cancer or any tumor.
- •
- has diabetes.
- •
- is pregnant or plans to become pregnant. It is not known if NGENLA will harm your child’s unborn baby. Talk to your child’s healthcare provider if your child is pregnant or plans to become pregnant.
- •
- is breastfeeding or plans to breastfeed. It is not known if NGENLA passes into breast milk. You and your child’s healthcare provider should decide if they will take NGENLA while breastfeeding.
Tell your child’s healthcare provider about all the medicines your child takes, including prescription and over-the-counter medicines, vitamins, and herbal supplements. NGENLA may affect how other medicines work, and other medicines may affect how NGENLA works.
How should I use NGENLA?
- •
- Read the detailed Instructions for Use that come with NGENLA.
- •
- NGENLA comes in 2 different dosage strengths. Your child’s healthcare provider will prescribe the dose of medicine and the dosage strength that is right for your child.
- •
- Your child’s healthcare provider will show you how to inject NGENLA before it is used for the first time. Do not try to inject NGENLA until you have been shown the right way by your child’s healthcare provider.
- •
- Inject NGENLA exactly as your child’s healthcare provider tells you to.
- •
- NGENLA is injected under the skin (subcutaneously) and can be given in the stomach (abdomen), thighs, buttocks, or upper arms. Rotate the site of injection weekly.
- •
- Inject NGENLA 1 time each week, on the same day each week, at any time of the day.
- •
- You may change the day of the week NGENLA is used as long as the last dose was injected 3 or more days before. After the new day of the week has been chosen, continue with the 1 time a week schedule.
- •
- If your child misses a dose of NGENLA the missed dose should be injected as soon as possible within 3 days after the missed dose. If more than 3 days have passed, skip your child’s missed dose. Inject the next dose on the regularly scheduled day.
- •
- NGENLA prefilled pens are for use by 1 person only.
- •
- Do not share your child’s NGENLA prefilled pens and needles with another person, even if the needle has been changed. Your child may give another person an infection or get an infection from them.
What are the possible side effects of NGENLA?
NGENLA may cause serious side effects, including:
- •
- high risk of death in people who have critical illnesses because of heart or stomach surgery, trauma or serious breathing (respiratory) problems.
- •
- serious allergic reactions. Get medical help right away if your child has the following symptoms:
- o
- swelling of the face, lips, mouth, or tongue
- o
- trouble breathing
- o
- wheezing
- o
- severe itching
- o
- skin rashes, redness, or swelling
- o
- dizziness or fainting
- o
- fast heartbeat or pounding in the chest
- o
- sweating
- •
- increased risk of growth of cancer or a tumor that is already present and increased risk of the return of cancer or a tumor in people who were treated with radiation to the brain or head as children and who developed low growth hormone problems. Your child’s healthcare provider will need to monitor your child for a return of cancer or a tumor. Contact your child’s healthcare provider if your child starts to have headaches, or has changes in behavior, changes in vision, or changes in moles, birthmarks, or the color of the skin.
- •
- new or worsening high blood sugar (hyperglycemia) or diabetes. Your child’s blood sugar may need to be monitored during treatment with NGENLA.
- •
- increase in pressure inside the skull (intracranial hypertension). If your child has headaches, eye problems, nausea or vomiting, contact your child’s healthcare provider.
- •
- your child’s body holding too much fluid (fluid retention) such as swelling in the hands and feet, pain in the joints or muscles or nerve problems that cause pain, burning or tingling in the hands, arms, legs and feet. Fluid retention can happen in children during treatment with NGENLA. Tell your child’s healthcare provider if your child has any of these signs or symptoms of fluid retention.
- •
- decrease in a hormone called cortisol. Your child’s healthcare provider will do blood tests to check your child’s cortisol levels. Tell your child’s healthcare provider if your child has severe fatigue, dizziness, weakness, vomiting, dehydration, or weight loss.
- •
- decrease in thyroid hormone levels. Decreased thyroid hormone levels may affect how well NGENLA works. Your child’s healthcare provider will do blood tests to check your child’s hormone levels.
- •
- hip and knee pain or limp (slipped capital femoral epiphysis). Tell your child’s healthcare provider if your child has any of these signs or symptoms.
- •
- worsening of curvature of the spine (scoliosis). If your child has scoliosis, your child will need to be checked often for an increase in the curve of the spine.
- •
- severe and constant stomach (abdominal) pain. This could be a sign of pancreatitis. Tell your child’s healthcare provider if your child has any new abdominal pain.
- •
- loss of fat and tissue weakness in the area of skin you inject. Talk to your child’s healthcare provider about rotating the areas where you inject NGENLA.
- •
- high risk of sudden death in children with Prader-Willi syndrome who are severely obese or have breathing problems, including sleep apnea.
- •
- increase in phosphorus, alkaline phosphatase and parathyroid hormone levels in the blood. Your child’s healthcare provider will do blood tests to check this.
The most common side effects of NGENLA include:
-
- o
- injection site reactions (such as pain, swelling, rash, itching, bleeding)
- o
- common cold
- o
- headache
- o
- fever
- o
- low red blood cells (anemia)
- o
- cough
- o
- vomiting
- o
- decrease in thyroid hormone levels
- o
- stomach (abdominal) pain
- o
- rash
- o
- throat pain
These are not all the possible side effects of NGENLA. You should tell your child’s healthcare provider if your child has any side effect that bothers them or that does not go away.
Call your child’s doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store NGENLA?
- •
- Do not freeze your pen or expose it to heat.
- •
- Do not use your pen if it has been frozen or stored in direct sunlight.
- •
- Do not use after the expiration date printed on the label has passed or if it has been more than 28 days after first use.
Before you use NGENLA pens for the first time (unused pens):
- •
- Store in the original carton.
- •
- Store the new, unused NGENLA pen in the refrigerator between 36°F to 46°F (2°C to 8°C).
- •
- Unused prefilled pens may be used until the expiration date printed on the pen label, if kept in the refrigerator.
After you use NGENLA pens and there is still medicine left (up to 28 days of use):
- •
- To help you remember when to dispose of (throw away) the pen, write the date of first use on the pen label.
- •
- Store remaining NGENLA in the refrigerator between 36°F to 46°F (2°C to 8°C) and use within 28 days.
- •
- Keep the pen cap on the NGENLA pen when it is not in use.
- •
- Do not store the remaining NGENLA pen with a needle attached.
- •
- Do not use the NGENLA pen if it has been more than 28 days after first use, even if it contains unused medicine.
Keep NGENLA and all medicines out of the reach of children.
General information about the safe and effective use of NGENLA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use NGENLA for a condition for which it was not prescribed. Do not give NGENLA to other people, even if they have the same symptoms because it may harm them. You can ask your pharmacist or healthcare provider for information about NGENLA that is written for health professionals.
What are the ingredients in NGENLA?
Active ingredient: somatrogon-ghla
Inactive ingredients: citric acid monohydrate, histidine, metacresol (as a preservative), poloxamer 188, sodium chloride, sodium citrate, water for injection.
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
Manufactured by:
Pfizer Ireland Pharmaceuticals
Ringaskiddy,
Cork,
Ireland
US License No: 2060

LAB-1449-1.0
For more information, go to website www.NGENLA.com or call 1-800-438-1985.This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 6/2023
-
Instructions for Use
NGENLA® (en’ JEN-lah)
(somatrogon-ghla)
24 mg
injection, for subcutaneous use
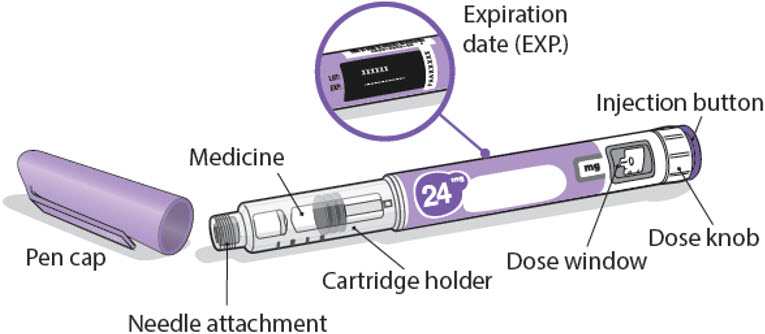
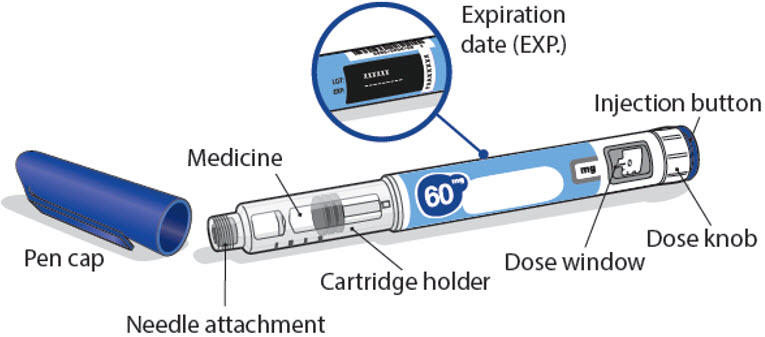
Read this Instructions for Use before you start using NGENLA and each time you get a new refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.Important information about your NGENLA pen:
- •
- The NGENLA pen for injection is a single-patient-use, disposable (throw away) prefilled pen containing 24 mg of medicine. You can give more than 1 dose from the pen.
- •
- NGENLA can be given by a patient, caregiver or healthcare provider. Do not try to inject NGENLA yourself until you are shown the right way to give the injections and read and understand the Instructions for Use. If your healthcare provider decides that you or a caregiver may be able to give your injections of NGENLA at home, you should receive training on the right way to prepare and inject NGENLA. It is important that you read, understand, and follow these instructions so that you inject NGENLA the right way.
- •
- It is important to talk to your healthcare provider to be sure you understand your NGENLA dosing instructions. To help you remember when to inject NGENLA, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject NGENLA, or call the helpline on 1-800-645-1280.
- •
- Do not share your pen with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
- •
- Each turn (click) of the dose knob dials 0.2 mg of medicine. You can give from 0.2 mg to 12 mg in a single injection. If your dose is more than 12 mg, you will need to give more than 1 injection.
- •
- A new pen may contain slightly more than 24 mg of medicine, this is normal.
- •
- Always use a new sterile needle for each injection. This will decrease the risk of contamination, infection, leakage of medicine, and blocked needles leading to the wrong dose.
- •
- Do not shake your pen. Shaking can damage the medicine.
- •
- The pen is not recommended for use by the blind or visually impaired without the assistance of a person trained in the proper use of the product.
Supplies you will need each time you inject
Included in the carton:
- •
- 1 NGENLA prefilled pen
Not included in the carton:
- •
- 1 new sterile needle for each injection
- •
- Alcohol swabs
- •
- Cotton balls or gauze pads
- •
- Adhesive bandage
- •
- 1 FDA-cleared sharps disposal container for disposal of pen needles and pens (See How should I dispose of the pen needles and pens?)
24 mg NGENLA pen:
Needles to use
Pen needles are not included with your NGENLA pen. You will need a prescription from your healthcare provider to get pen needles up to a length of 8 mm from your pharmacy.
- •
- Needles to use with your NGENLA pen:
- o
- 32G (Novo Nordisk®, NovoFine® Plus)
- o
- 31G (Novo Nordisk®, NovoFine®)
- o
- 31G (Becton Dickinson and Company, BD Ultra-Fine™ or BD Micro-Fine™)
- •
- Talk with your healthcare provider about the right needle for you.
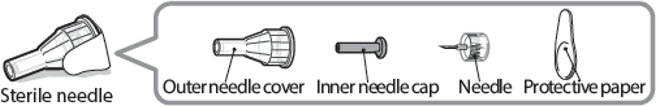
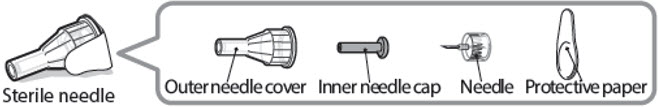
Sterile needle (example) not supplied:
Caution: Never use a bent or damaged needle. Always handle pen needles with care to make sure you do not prick yourself (or anyone else) with the needle. Do not attach a new needle to your pen until you are ready to take your injection.
Preparing for your injection
Step 1 - Getting ready
- •
- Wash and dry your hands.
- •
- You can use your pen straight from the refrigerator. For a more comfortable injection, leave your pen at room temperature for up to 30 minutes.
- •
- Check the name, strength, and label of your pen to make sure it is the medicine your healthcare provider has prescribed for you.
- •
- Check the expiration date on the pen label. Do not use if the expiration date has passed.
- •
-
Do not use your pen if:
- o
- it has been frozen or exposed to heat
- o
- it has been dropped
- o
- it looks broken or damaged
- o
- it has been more than 28 days after first use of the pen.
- •
- Do not remove the pen cap from your pen - until you are ready to inject.
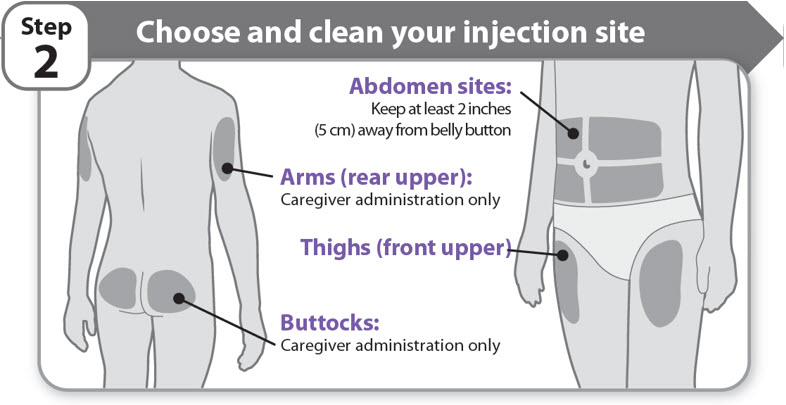
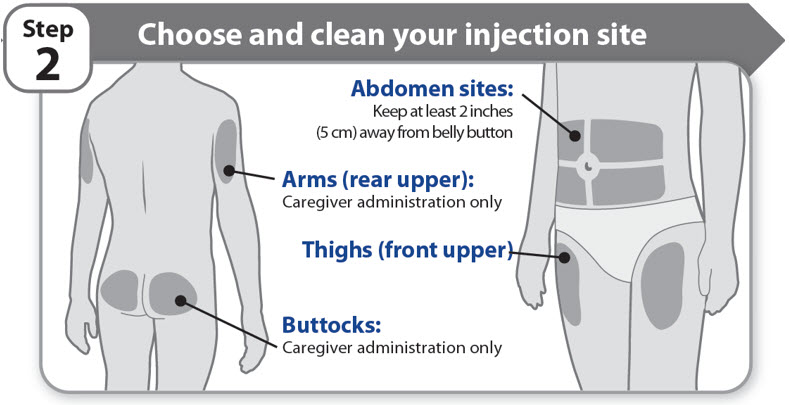
Step 2 - Choose and clean your injection site
- •
- NGENLA can be given in the abdomen, thighs, buttocks, or upper arms.
- •
- Choose the best place to inject, as recommended by your healthcare provider.
- •
- If more than 1 injection is needed to complete your full dose, each injection should be given in a different injection site.
- •
- Do not inject into bony areas, areas that are bruised, red, sore or hard, and areas that have scars or skin conditions.
- •
- Clean the injection site with an alcohol swab.
- •
- Allow the injection site to dry.
- •
- Do not touch injection site after cleaning.
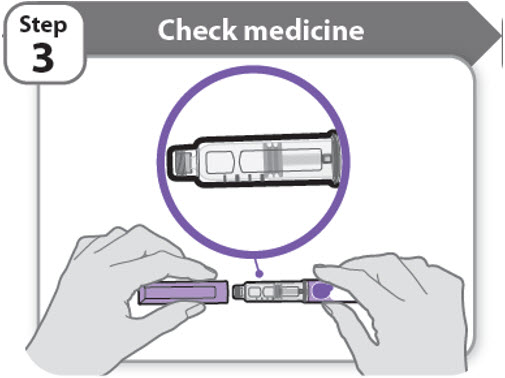
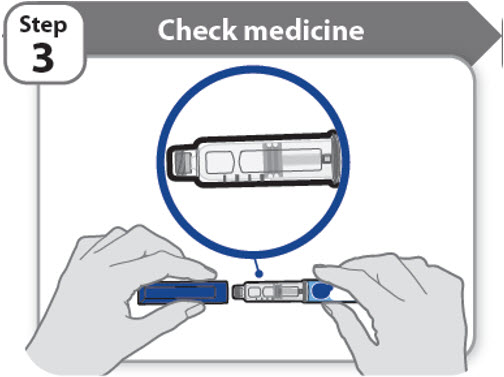
Step 3 - Check medicine
- •
- Pull off the pen cap and keep it for after your injection.
- •
- Check the medicine inside the cartridge holder.
- •
- Make sure the medicine is colorless to slightly light yellow. Do not inject the medicine if it is cloudy or dark yellow.
- •
- Make sure the medicine is free of flakes or particles. Do not inject the medicine if it has flakes or particles.
- •
- Note: It is normal to see one or more bubbles in the medicine.
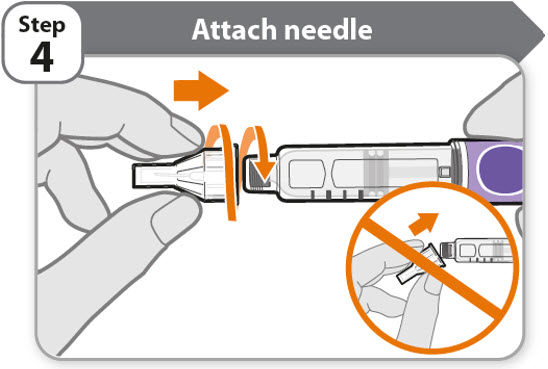
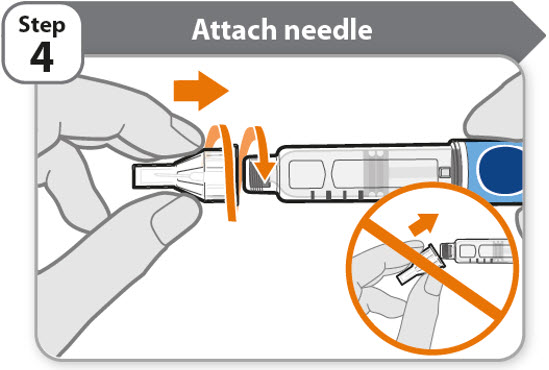
- •
- Take a new needle and pull off the protective paper.
- •
- Line the needle up with your pen keeping them both straight.
- •
- Gently push and then screw the needle onto your pen.
Do not over tighten.
Note: Be careful not to attach the needle at an angle. This may cause the pen to leak.
Caution: Needles have sharp tips at both ends. Handle with care to make sure you do not prick yourself (or anyone else) with the needle.
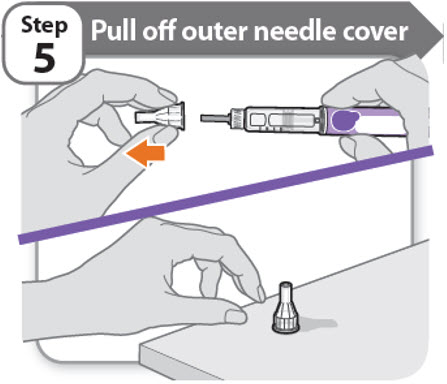
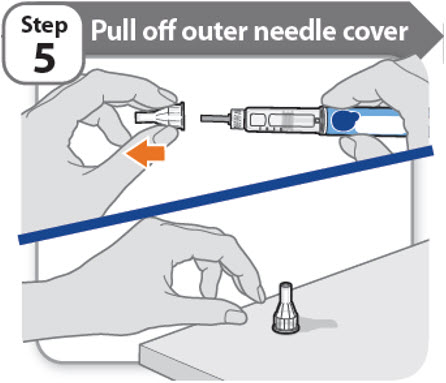
Step 5 - Pull off outer needle cover
- •
- Pull off the outer needle cover.
- •
- Make sure you keep the outer needle cover. You will need it later to remove the needle.
Note: You should see an inner needle cap after you have removed the outer cover. If you do not see this, try to attach the needle again.
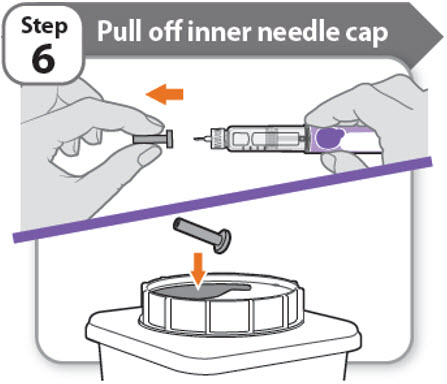
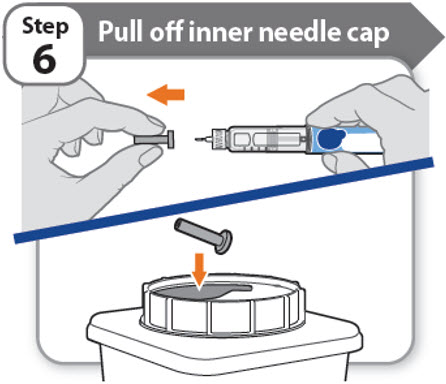
Step 6 - Pull off inner needle cap
- •
- Pull off the inner needle cap carefully to show the needle.
- •
- Throw away the inner needle cap in a FDA-cleared sharps container. It is not needed again.
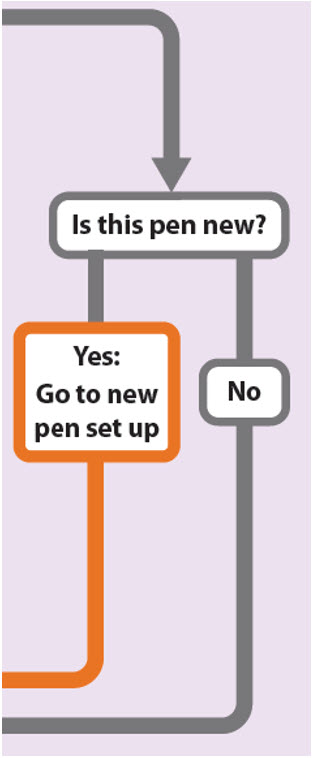
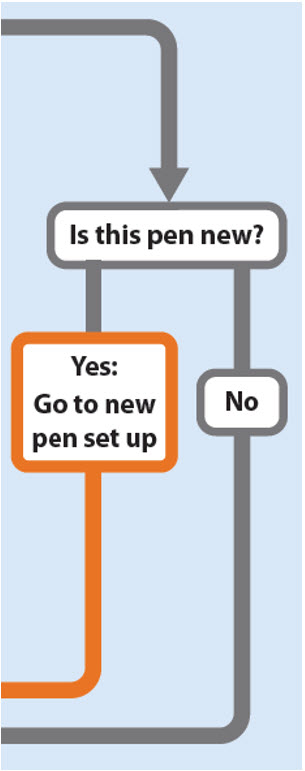
Is this pen new?
Yes: Go to new pen set up
No
New pen set up (priming) – for the first use of a new pen only
You must set up each new pen (priming) before using it for the first time
- •
- New pen set up is done before each new pen is used for the first time.
- •
- The purpose of setting up a new pen is to remove air bubbles and make sure you get the correct dose.
Important: Skip Step-A through to Step-C if you have already set up your pen.
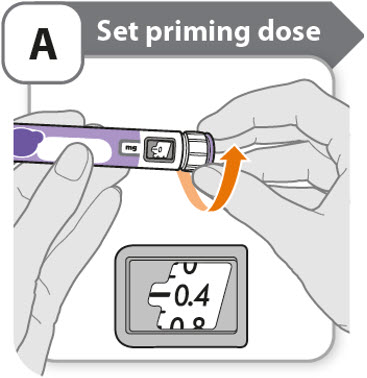
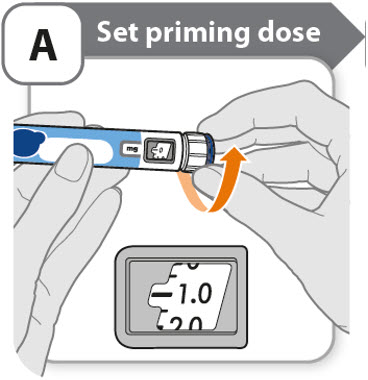
A - Set priming dose
- •
- Turn the dose knob to 0.4.
- o
- This is the amount to prime the pen.
- Note: If you turn the dose knob too far, you can turn it back.
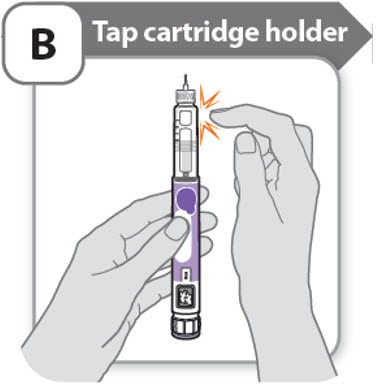
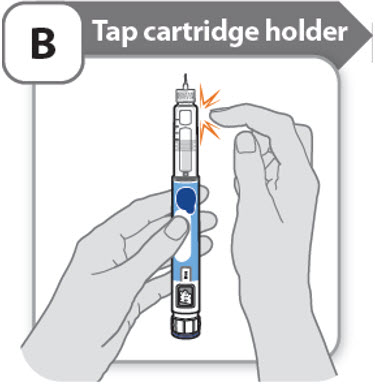
B - Tap cartridge holder
- •
- Hold the pen with the needle pointing up so that the air bubbles can rise.
- •
-
Tap the cartridge holder gently to float any air bubbles to the top.
Important: Follow Step-B even if you do not see air bubbles.
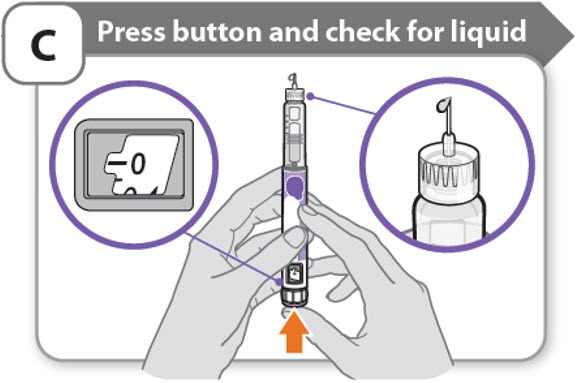
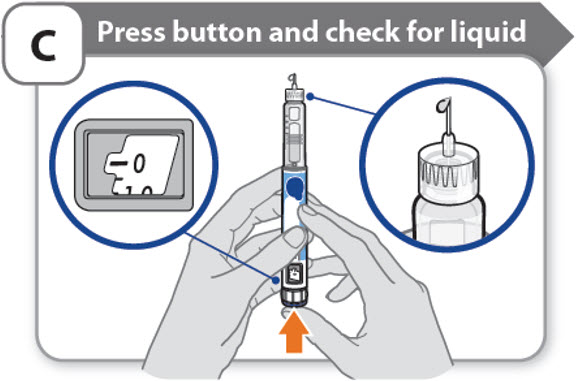
C - Press button and check for liquid
- •
- Press the injection button until it cannot go any further and "0" is shown in the dose window.
- •
- Check for liquid at the needle tip. If liquid appears, your pen is set up.
- •
- Always make sure that a drop of liquid appears before you inject. If liquid has not appeared, repeat Step-A through to Step-C.
- o
- If liquid does not appear after you have repeated Step-A through Step-C five (5) times, attach a new needle and try 1 more time. Do not use the pen if a drop of liquid still does not appear. Contact your healthcare provider or pharmacist and use a new pen.
Setting your prescribed dose
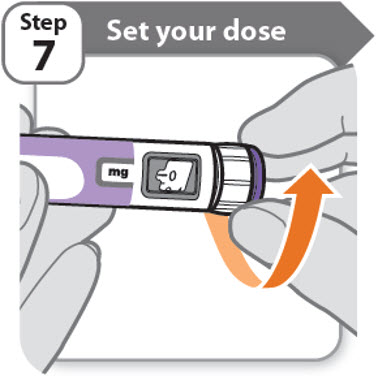
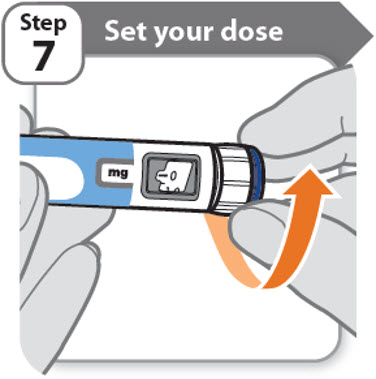
Step 7 - Set your dose
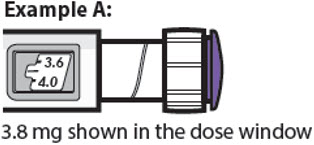
3.8 mg shown in the dose window
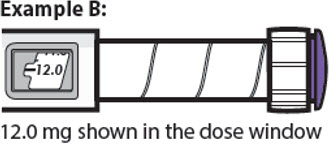
12.0 mg shown in the dose window
- •
- Turn the dose knob to set your dose.
- o
- The dose can be increased or decreased by turning the dose knob in either direction.
- o
- The dose knob turns 0.2 mg at a time.
- o
- Your pen contains 24 mg of medicine but you can only set a dose of up to 12 mg for a single injection.
- o
- The dose window shows the dose in mg. See Examples A and B.
- •
-
Always check the dose window to make sure you have set the correct dose.
Important: Do not press the injection button while setting your dose.
What should I do if I cannot set the dose I need?
- •
- If your dose is more than 12 mg you will need to split your dose into more than 1 injection.
- •
- You can give from 0.2 mg to 12 mg in a single injection.
- o
- Use a new needle for each injection (See Step 4: Attach needle).
- o
- If you normally need to give 2 injections for your full dose, be sure to give your second dose.
What should I do if I do not have enough medicine left in my pen?
- •
- If your pen contains less than 12 mg of medicine, the dose knob will stop with the remaining amount of medicine shown in the dose window.
- •
- If there is not enough medicine left in your pen for your full dose, you may either:
- o
- Inject the amount left in your pen, then prepare a new pen to complete your dose in full. Remember to subtract the dose you have already received. For example, if the dose is 3.8 mg and you can only set the dose knob to 1.8 mg, you should inject another 2.0 mg with a new pen.
- o
- Or get a new pen and inject the full dose.
Only split your dose if you have been trained or told by your healthcare provider on how to do this.
Injecting your dose
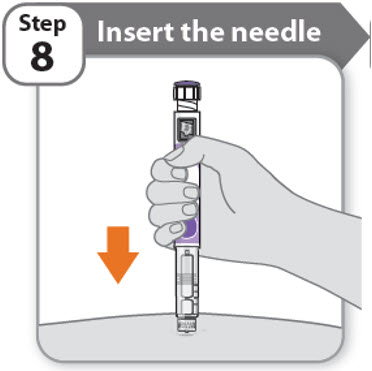
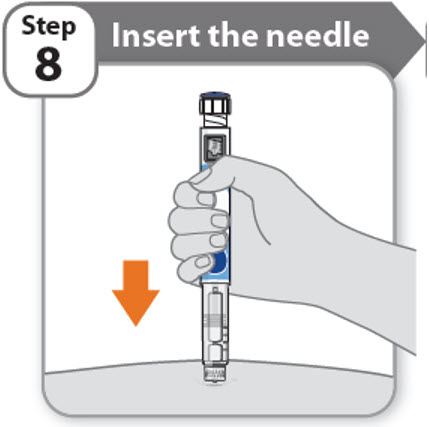
Step 8 - Insert the needle
- •
- Hold your pen so you can see the numbers in the dose window.
- •
- Insert the needle straight into your skin.
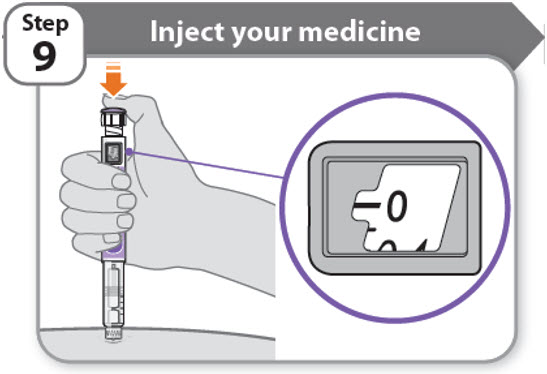
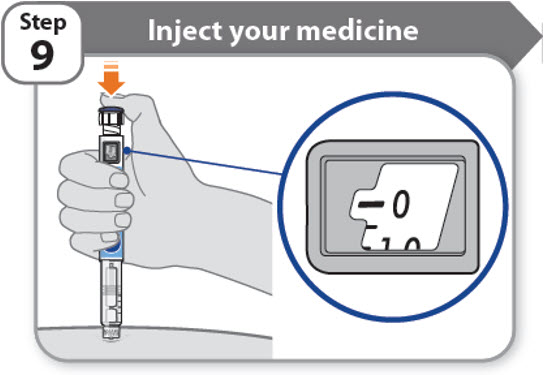
Step 9 - Inject your medicine
- •
- Keep holding the needle in the same position in your skin.
- •
- Press the injection button until it cannot go any further and "0" is shown in the dose window.
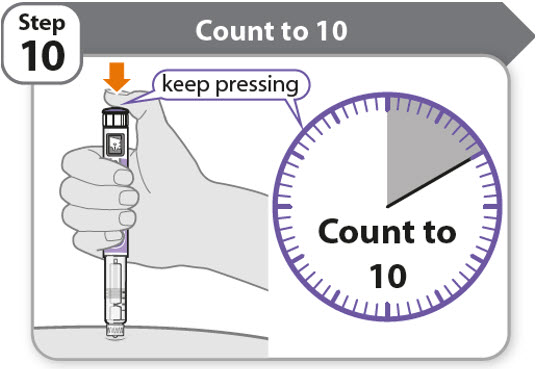
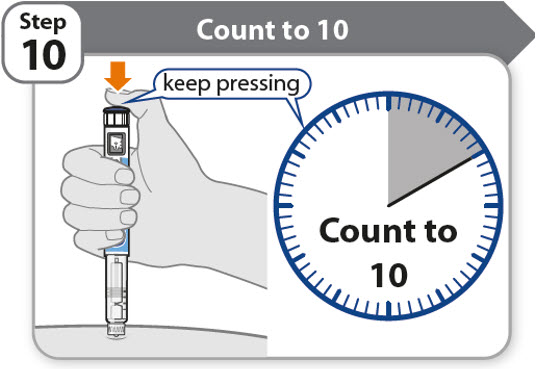
Step 10 - Count to 10
- •
- Continue to press the injection button while counting to 10. Counting to 10 will allow the full dose of medicine to be given.
- •
- After counting to 10, let go of the injection button and slowly remove the pen from the injection site by pulling the needle straight out.
Note: You may see a drop of medicine at the needle tip. This is normal and does not affect the dose you just received.
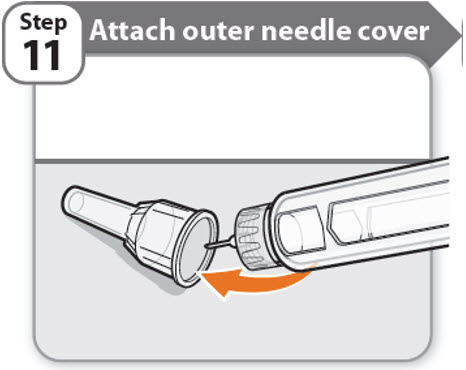
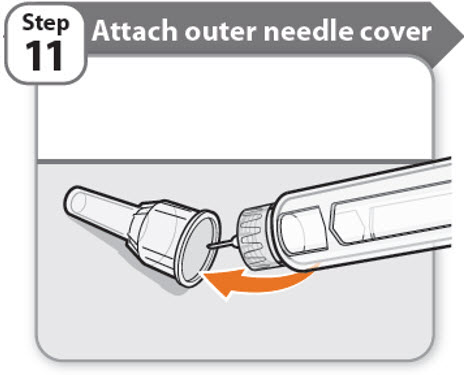
Step 11 - Attach outer needle cover
- •
- Carefully place the outer needle cover back on the needle.
- •
- Press on the outer needle cover until it is secure.
Caution: Never try to put the inner needle cap back on the needle. You may prick yourself with the needle.
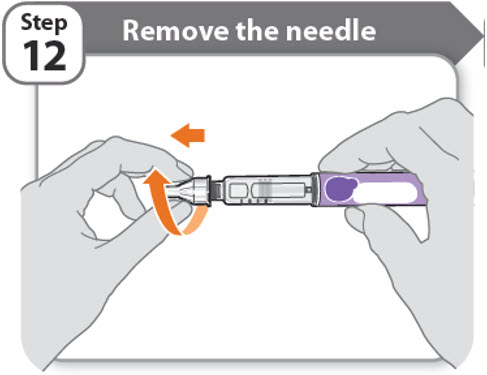
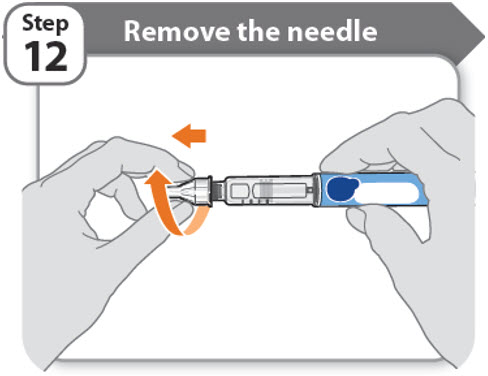
Step 12 - Remove the needle
- •
- Unscrew the capped needle from the pen.
- •
- Gently pull until the capped needle comes off.
Note: If the needle is still on, replace the outer needle cover and try again. Be sure to apply pressure when unscrewing the needle. - •
- Throw away the needle in the FDA-cleared sharps container (See How should I dispose of the pen needles and pens?).
- •
- Important: Always remove and throw away used needles. Do not reuse needles.
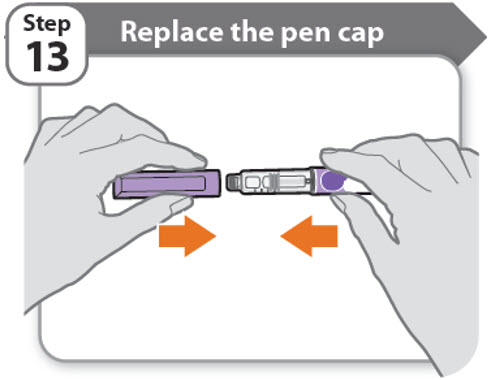
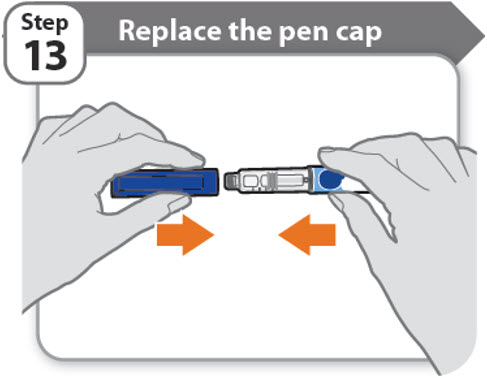
Step 13 - Replace the pen cap
- •
- Replace the pen cap back onto your pen.
- •
- Do not recap the pen with a needle attached.
- •
- If there is any medicine left in your pen, store in the refrigerator between uses (See How should I store my pen?).
Step 14 - After your injection
- •
- Press lightly on the injection site with a clean cotton ball or gauze pad, and hold for a few seconds.
- •
- Do not rub the injection site. You may have slight bleeding. This is normal.
- •
- You may cover the injection site with a small adhesive bandage, if needed.
- •
- If your pen is empty or it has been more than 28 days after first use, throw it away even if it contains unused medicine. See Storage and disposal on the right side of this leaflet.
Storage and disposal:
- •
- Do not freeze your pen or expose it to heat.
- •
- Do not use your pen if it has been frozen or stored in direct sunlight.
- •
- Do not use after the expiration date printed on the label has passed or if it has been more than 28 days after first use.
- •
- Keep NGENLA and all medicines out of the reach of children.
Before first use (unused pens):
- •
- Store in the original carton.
- •
- Store your pens in the refrigerator between 36°F to 46°F (2°C to 8°C).
- •
- Unused pens may be used until the expiration date printed on the label, only if the pen has been kept in the refrigerator.
After first use (up to 28 days of use):
- •
- Store your pen in the refrigerator between 36°F to 46°F (2°C to 8°C) and away from direct sunlight.
- •
- Keep the pen cap on your pen when it is not in use.
- •
- Do not store the pen with a needle attached.
- •
- If your pen is empty or it has been more than 28 days after first use, throw it away even if it contains unused medicine.
- •
- To help you remember when to dispose of your pen you can write the date of first use on the pen label and below:
Date of first use ______ / ______ / ______
How should I dispose of the pen needles and pens?
- •
- Throw away your pen, and pen needles into a FDA-cleared sharps disposal container or puncture resistant container.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that:
- o
- is made of heavy-duty plastic
- o
- can be closed with a tight-fitting, puncture resistant lid, without sharps being able to come out
- o
- is upright and stable during use
- o
- is leak-resistant, and properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles, syringes, and prefilled syringes.
- •
- For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: https://www.fda.gov/safesharpsdisposal
- •
- Do not throw away your used sharps disposal container in your household trash unless your community guidelines permit this.
- •
- Keep the sharps container out of the reach of children.
Manufactured by:
Pfizer Ireland Pharmaceuticals
Ringaskiddy,
Cork,
IrelandUS License No: 2060
LAB-1452-1.0
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 6/2023
-
INSTRUCTIONS FOR USE
Instructions for Use
NGENLA® (en’ JEN-lah)(somatrogon-ghla)
60 mg
injection, for subcutaneous use
Read this Instructions for Use before you start using NGENLA and each time you get a new refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.Important information about your NGENLA pen:
- •
- The NGENLA pen for injection is a single-patient-use, disposable (throw away) prefilled pen containing 60 mg of medicine. You can give more than 1 dose from the pen.
- •
- NGENLA can be given by a patient, caregiver or healthcare provider. Do not try to inject NGENLA yourself until you are shown the right way to give the injections and read and understand the Instructions for Use. If your healthcare provider decides that you or a caregiver may be able to give your injections of NGENLA at home, you should receive training on the right way to prepare and inject NGENLA. It is important that you read, understand, and follow these instructions so that you inject NGENLA the right way.
- •
- It is important to talk to your healthcare provider to be sure you understand your NGENLA dosing instructions. To help you remember when to inject NGENLA, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject NGENLA, or call the helpline on 1-800-645-1280.
- •
- Do not share your pen with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
- •
- Each turn (click) of the dose knob dials 0.5 mg of medicine. You can give from 0.5 mg to 30 mg in a single injection. If your dose is more than 30 mg, you will need to give more than 1 injection.
- •
- A new pen may contain slightly more than 60 mg of medicine, this is normal.
- •
- Always use a new sterile needle for each injection. This will decrease the risk of contamination, infection, leakage of medicine, and blocked needles leading to the wrong dose.
- •
- Do not shake your pen. Shaking can damage the medicine.
- •
- The pen is not recommended for use by the blind or visually impaired without the assistance of a person trained in the proper use of the product.
Supplies you will need each time you inject
Included in the carton:
- •
- 1 NGENLA prefilled pen
Not included in the carton:
- •
- 1 new sterile needle for each injection
- •
- Alcohol swabs
- •
- Cotton balls or gauze pads
- •
- Adhesive bandage
- •
- 1 FDA-cleared sharps disposal container for disposal of pen needles and pens (See How should I dispose of the pen needles and pens?)
60 mg NGENLA pen:
Needles to use
Pen needles are not included with your NGENLA pen. You will need a prescription from your healthcare provider to get pen needles up to a length of 8 mm from your pharmacy.
- •
- Needles to use with your NGENLA pen:
- o
- 32G (Novo Nordisk®, NovoFine® Plus)
- o
- 31G (Novo Nordisk®, NovoFine®)
- o
- 31G (Becton Dickinson and Company, BD Ultra-Fine™ or BD Micro-Fine™)
- •
- Talk with your healthcare provider about the right needle for you.
Sterile needle (example) not supplied:
Caution: Never use a bent or damaged needle. Always handle pen needles with care to make sure you do not prick yourself (or anyone else) with the needle. Do not attach a new needle to your pen until you are ready to take your injection.
Preparing for your injection
Step 1 - Getting ready
- •
- Wash and dry your hands.
- •
- You can use your pen straight from the refrigerator. For a more comfortable injection, leave your pen at room temperature for up to 30 minutes.
- •
- Check the name, strength, and label of your pen to make sure it is the medicine your healthcare provider has prescribed for you.
- •
- Check the expiration date on the pen label. Do not use if the expiration date has passed.
- •
-
Do not use your pen if:
- o
- it has been frozen or exposed to heat
- o
- it has been dropped
- o
- it looks broken or damaged
- o
- it has been more than 28 days after first use of the pen.
- •
- Do not remove the pen cap from your pen - until you are ready to inject.
Step 2 - Choose and clean your injection site
- •
- NGENLA can be given in the abdomen, thighs, buttocks, or upper arms.
- •
- Choose the best place to inject, as recommended by your healthcare provider.
- •
- If more than 1 injection is needed to complete your full dose, each injection should be given in a different injection site.
- •
- Do not inject into bony areas, areas that are bruised, red, sore or hard, and areas that have scars or skin conditions.
- •
- Clean the injection site with an alcohol swab.
- •
- Allow the injection site to dry.
- •
- Do not touch injection site after cleaning.
Step 3 - Check medicine
- •
- Pull off the pen cap and keep it for after your injection.
- •
- Check the medicine inside the cartridge holder.
- •
- Make sure the medicine is colorless to slightly light yellow. Do not inject the medicine if it is cloudy or dark yellow.
- •
- Make sure the medicine is free of flakes or particles. Do not inject the medicine if it has flakes or particles.
Note: It is normal to see one or more bubbles in the medicine.
- •
- Take a new needle and pull off the protective paper.
- •
- Line the needle up with your pen keeping them both straight.
- •
- Gently push and then screw the needle onto your pen.
Do not over tighten.
Note: Be careful not to attach the needle at an angle. This may cause the pen to leak.
Caution: Needles have sharp tips at both ends. Handle with care to make sure you do not prick yourself (or anyone else) with the needle.
Step 5 - Pull off outer needle cover
- •
- Pull off the outer needle cover.
- •
- Make sure you keep the outer needle cover. You will need it later to remove the needle.
Note: You should see an inner needle cap after you have removed the outer cover. If you do not see this, try to attach the needle again.
Step 6 - Pull off inner needle cap
- •
- Pull off the inner needle cap carefully to show the needle.
- •
- Throw away the inner needle cap in a FDA-cleared sharps container. It is not needed again.
Is this pen new?
Yes: Go to new pen set up
No
New pen set up (priming) – for the first use of a new pen only
You must set up each new pen (priming) before using it for the first time
- •
- New pen set up is done before each new pen is used for the first time.
- •
- The purpose of setting up a new pen is to remove air bubbles and make sure you get the correct dose.
Important: Skip Step-A through to Step-C if you have already set up your pen.
A - Set priming dose
- •
- Turn the dose knob to 1.0.
- o
- This is the amount to prime the pen.
- Note: If you turn the dose knob too far, you can turn it back.
B - Tap cartridge holder
- •
- Hold the pen with the needle pointing up so that the air bubbles can rise.
- •
-
Tap the cartridge holder gently to float any air bubbles to the top.
Important: Follow Step-B even if you do not see air bubbles.
C - Press button and check for liquid
- •
- Press the injection button until it cannot go any further and "0" is shown in the dose window.
- •
- Check for liquid at the needle tip. If liquid appears, your pen is set up.
- •
- Always make sure that a drop of liquid appears before you inject. If liquid has not appeared, repeat Step-A through to Step-C.
- o
- If liquid does not appear after you have repeated Step-A through Step-C five (5) times, attach a new needle and try 1 more time. Do not use the pen if a drop of liquid still does not appear. Contact your healthcare provider or pharmacist and use a new pen.
Setting your prescribed dose
Step 7 - Set your dose
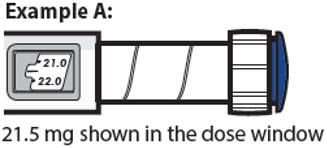
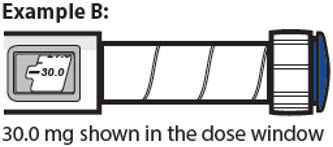
21.5 mg shown in the dose window
30.0 mg shown in the dose window
- •
- Turn the dose knob to set your dose.
- o
- The dose can be increased or decreased by turning the dose knob in either direction.
- o
- The dose knob turns 0.5 mg at a time.
- o
- Your pen contains 60 mg of medicine but you can only set a dose of up to 30 mg for a single injection.
- o
- The dose window shows the dose in mg. See Examples A and B.
- •
-
Always check the dose window to make sure you have set the correct dose.
Important: Do not press the injection button while setting your dose.
What should I do if I cannot set the dose I need?
- •
- If your dose is more than 30 mg you will need to split your dose into more than 1 injection.
- •
- You can give from 0.5 mg to 30 mg in a single injection.
- o
- Use a new needle for each injection (See Step 4: Attach needle).
- o
- If you normally need to give 2 injections for your full dose, be sure to give your second dose.
What should I do if I do not have enough medicine left in my pen?
- •
- If your pen contains less than 30 mg of medicine, the dose knob will stop with the remaining amount of medicine shown in the dose window.
- •
- If there is not enough medicine left in your pen for your full dose, you may either:
- o
- Inject the amount left in your pen, then prepare a new pen to complete your dose in full. Remember to subtract the dose you have already received. For example, if the dose is 21.5 mg and you can only set the dose knob to 17 mg, you should inject another 4.5 mg with a new pen.
- o
- Or get a new pen and inject the full dose.
Only split your dose if you have been trained or told by your healthcare provider on how to do this.
Injecting your dose
Step 8 - Insert the needle
- •
- Hold your pen so you can see the numbers in the dose window.
- •
- Insert the needle straight into your skin.
Step 9 - Inject your medicine
- •
- Keep holding the needle in the same position in your skin.
- •
- Press the injection button until it cannot go any further and "0" is shown in the dose window.
Step 10 - Count to 10
- •
- Continue to press the injection button while counting to 10. Counting to 10 will allow the full dose of medicine to be given.
- •
- After counting to 10, let go of the injection button and slowly remove the pen from the injection site by pulling the needle straight out.
Note: You may see a drop of medicine at the needle tip. This is normal and does not affect the dose you just received.
Step 11 - Attach outer needle cover
- •
- Carefully place the outer needle cover back on the needle.
- •
- Press on the outer needle cover until it is secure.
Caution: Never try to put the inner needle cap back on the needle. You may prick yourself with the needle.
Step 12 - Remove the needle
- •
- Unscrew the capped needle from the pen.
- •
- Gently pull until the capped needle comes off.
Note: If the needle is still on, replace the outer needle cover and try again. Be sure to apply pressure when unscrewing the needle. - •
- Throw away the needle in the FDA-cleared sharps container (See How should I dispose of the pen needles and pens?).
- •
- Important: Always remove and throw away used needles. Do not reuse needles.
Step 13 - Replace the pen cap
- •
- Replace the pen cap back onto your pen.
- •
- Do not recap the pen with a needle attached.
- •
- If there is any medicine left in your pen, store in the refrigerator between uses (See How should I store my pen?).
Step 14 - After your injection
- •
- Press lightly on the injection site with a clean cotton ball or gauze pad, and hold for a few seconds.
- •
- Do not rub the injection site. You may have slight bleeding. This is normal.
- •
- You may cover the injection site with a small adhesive bandage, if needed.
- •
- If your pen is empty or it has been more than 28 days after first use, throw it away even if it contains unused medicine. See Storage and disposal on the right side of this leaflet.
Storage and disposal:
- •
- Do not freeze your pen or expose it to heat.
- •
- Do not use your pen if it has been frozen or stored in direct sunlight.
- •
- Do not use after the expiration date printed on the label has passed or if it has been more than 28 days after first use.
- •
- Keep NGENLA and all medicines out of the reach of children.
Before first use (unused pens):
- •
- Store in the original carton.
- •
- Store your pens in the refrigerator between 36°F to 46°F (2°C to 8°C).
- •
- Unused pens may be used until the expiration date printed on the label, only if the pen has been kept in the refrigerator.
After first use (up to 28 days of use):
- •
- Store your pen in the refrigerator between 36°F to 46°F (2°C to 8°C) and away from direct sunlight.
- •
- Keep the pen cap on your pen when it is not in use.
- •
- Do not store the pen with a needle attached.
- •
- If your pen is empty or it has been more than 28 days after first use, throw it away even if it contains unused medicine.
- •
- To help you remember when to dispose of your pen you can write the date of first use on the pen label and below:
Date of first use ______ / ______ / ______
How should I dispose of the pen needles and pens?
- •
- Throw away your pen, and pen needles into a FDA-cleared sharps disposal container or puncture resistant container.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that:
- o
- is made of heavy-duty plastic
- o
- can be closed with a tight-fitting, puncture resistant lid, without sharps being able to come out
- o
- is upright and stable during use
- o
- is leak-resistant, and properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles, syringes, and prefilled syringes.
- •
- For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: https://www.fda.gov/safesharpsdisposal
- •
- Do not throw away your used sharps disposal container in your household trash unless your community guidelines permit this.
- •
- Keep the sharps container out of the reach of children.
Manufactured by:
Pfizer Ireland Pharmaceuticals
Ringaskiddy,
Cork,
IrelandUS License No: 2060
LAB-1454-1.0
This Instructions for Use has been approved by the U.S. Food and Drug Administration Issued: 6/2023
- PRINCIPAL DISPLAY PANEL - 24 mg Cartridge Label
-
PRINCIPAL DISPLAY PANEL - 24 mg Cartridge Carton
One single-patient-use
disposable prefilled pen
Rx only
NDC 0069-0505-0224mg
Ngenla®
(somatrogon-ghla)
Injection
24 mg/1.2 mL (20 mg/mL)
For Subcutaneous Injection OnlyREAD ENCLOSED INSTRUCTIONS BEFORE USE
Write the date of first use in the space provided on the
pen label. Discard the pen 28 days after first use, even if it
contains unused medicine.Before first use: Store refrigerated at 36°F to 46°F
(2°C to 8°C) in the original carton to protect from light.
After first use: Store refrigerated at 36°F to 46°F
(2°C to 8°C) between each use for up to 28 days. - PRINCIPAL DISPLAY PANEL - 60 mg Cartridge Label
-
PRINCIPAL DISPLAY PANEL - 60 mg Cartridge Carton
One single-patient-use
disposable prefilled pen
Rx only
NDC 0069-0520-0260mg
Ngenla®
(somatrogon-ghla)
Injection
60 mg/1.2 mL (50 mg/mL)
For Subcutaneous Injection OnlyREAD ENCLOSED INSTRUCTIONS BEFORE USE
Write the date of first use in the space provided on the
pen label. Discard the pen 28 days after first use, even if
it contains unused medicine.Before first use: Store refrigerated at 36°F to 46°F (2°C to 8°C)
in the original carton to protect from light. After first use: Store
refrigerated at 36°F to 46°F (2°C to 8°C) between each use for up
to 28 days. Do not freeze or shake. Do not expose to heat.
Store away from direct sunlight. Do not use if it has been frozen. -
INGREDIENTS AND APPEARANCE
NGENLA
somatrogon-ghla injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-0505 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOMATROGON (UNII: 6D848RA61B) (SOMATROGON - UNII:6D848RA61B) SOMATROGON 24 mg in 1.2 mL Inactive Ingredients Ingredient Name Strength TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) 3.1 mg in 1.2 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.3 mg in 1.2 mL HISTIDINE (UNII: 4QD397987E) 1.9 mg in 1.2 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 10 mg in 1.2 mL METACRESOL (UNII: GGO4Y809LO) 4 mg in 1.2 mL POLOXAMER 188 (UNII: LQA7B6G8JG) 2 mg in 1.2 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-0505-02 1 in 1 CARTON 07/31/2023 1 NDC:0069-0505-01 1.2 mL in 1 CARTRIDGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761184 07/31/2023 NGENLA
somatrogon-ghla injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-0520 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOMATROGON (UNII: 6D848RA61B) (SOMATROGON - UNII:6D848RA61B) SOMATROGON 60 mg in 1.2 mL Inactive Ingredients Ingredient Name Strength TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) 3.1 mg in 1.2 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.3 mg in 1.2 mL HISTIDINE (UNII: 4QD397987E) 1.9 mg in 1.2 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 10 mg in 1.2 mL METACRESOL (UNII: GGO4Y809LO) 4 mg in 1.2 mL POLOXAMER 188 (UNII: LQA7B6G8JG) 2 mg in 1.2 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-0520-02 1 in 1 CARTON 07/31/2023 1 NDC:0069-0520-01 1.2 mL in 1 CARTRIDGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761184 07/31/2023 Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) Establishment Name Address ID/FEI Business Operations Pfizer Ireland Pharmaceuticals 985586408 ANALYSIS(0069-0505, 0069-0520) , API MANUFACTURE(0069-0505, 0069-0520) Establishment Name Address ID/FEI Business Operations Pfizer Manufacturing Belgium NV 370156507 ANALYSIS(0069-0505, 0069-0520) , MANUFACTURE(0069-0505, 0069-0520) , PACK(0069-0505, 0069-0520) , LABEL(0069-0505, 0069-0520) Establishment Name Address ID/FEI Business Operations Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC 174350868 API MANUFACTURE(0069-0505, 0069-0520) , ANALYSIS(0069-0505, 0069-0520)