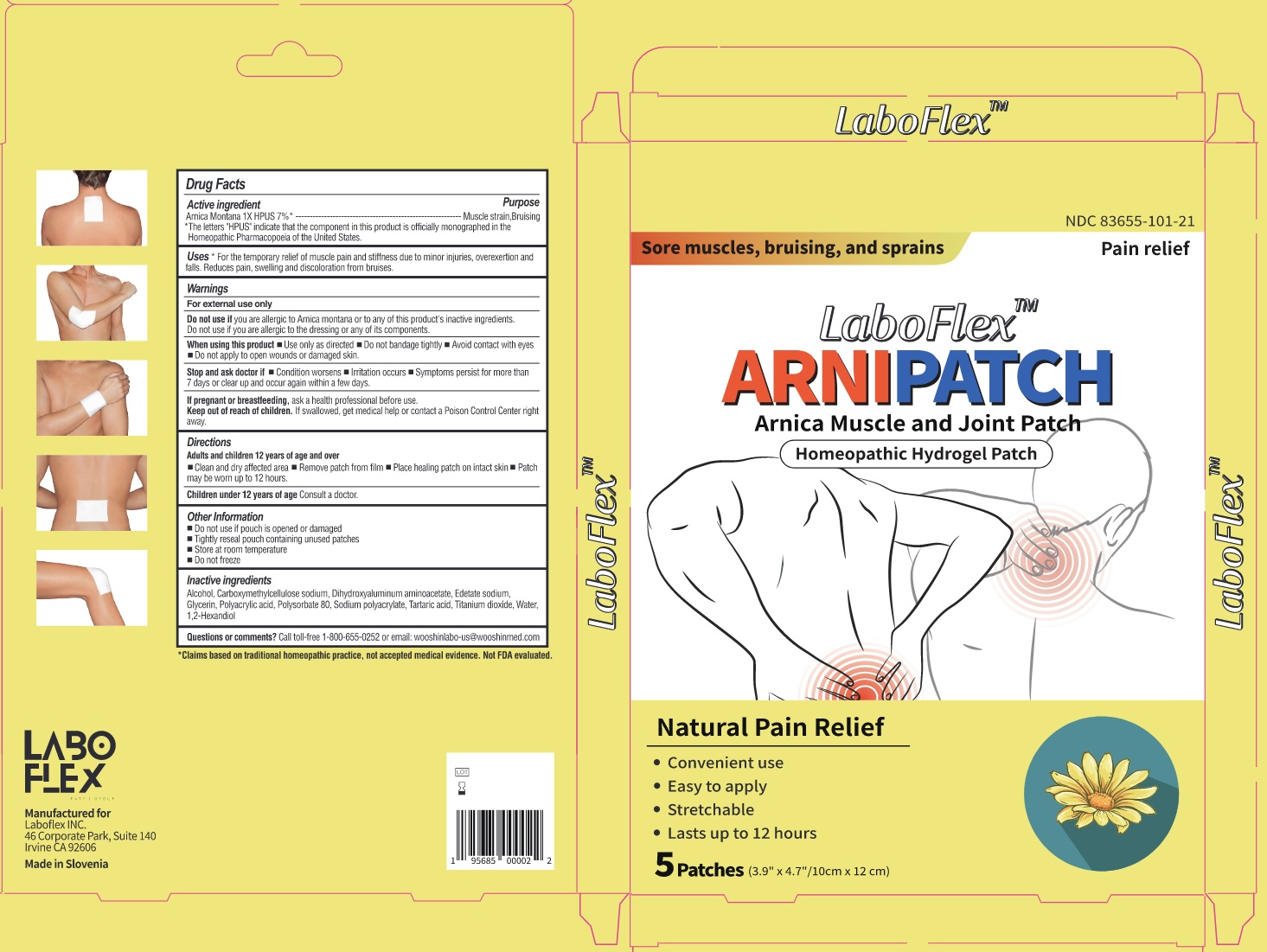
Label: LABOFLEX ARNIPATCH MUSCLE AND JOINT PATCH- arnica montana patch
- NDC Code(s): 83655-101-15, 83655-101-21
- Packager: Laboflex, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only.
Do not useif you are allergic to Arnica montana or to any of this product's inactive ingredients. Do not use if you are allergic to the dressing or any of its components.
When Using This Product
When using this product■ Use only as directed ■ Do not bandage tightly ■ Avoid contact with eyes ■ Do not apply to open wounds or damaged skin
Keep Out of Reach of Children
Keep out of reach of children. If swallowed, get medical help or contact a Posion Control Center right away.
- Directions
- Inactive Ingredients
- Questions of comments?
- Product Packaging
-
INGREDIENTS AND APPEARANCE
LABOFLEX ARNIPATCH MUSCLE AND JOINT PATCH
arnica montana patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83655-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYACRYLIC ACID (8000 MW) (UNII: 73861X4K5F) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) WATER (UNII: 059QF0KO0R) TARTARIC ACID (UNII: W4888I119H) ALCOHOL (UNII: 3K9958V90M) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) EDETATE SODIUM (UNII: MP1J8420LU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83655-101-15 5 g in 1 POUCH; Type 0: Not a Combination Product 09/07/2023 2 NDC:83655-101-21 5 in 1 CARTON 09/07/2023 2 5 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/07/2023 Labeler - Laboflex, Inc. (128394050) Registrant - Laboflex, Inc. (128394050) Establishment Name Address ID/FEI Business Operations WOOSHIN LAPACHE d.o.o. 507385209 manufacture(83655-101)