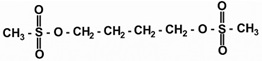Label: BUSULFAN injection, solution
- NDC Code(s): 55150-395-01, 55150-395-08
- Packager: Eugia US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BUSULFAN INJECTION safely and effectively. See full prescribing information for BUSULFAN INJECTION. BUSULFAN injection, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: MYELOSUPPRESSION
Busulfan injection causes severe and prolonged myelosuppression at the recommended dosage. Hematopoietic progenitor cell transplantation is required to prevent potentially fatal complications of the prolonged myelosuppression [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGEBusulfan injection is indicated for use in combination with cyclophosphamide as a conditioning regimen prior to allogeneic hematopoietic progenitor cell transplantation for chronic ...
-
2 DOSAGE AND ADMINISTRATION2.1 Initial Dosing Information - • Administer busulfan injection in combination with cyclophosphamide as a conditioning regimen prior to bone marrow or peripheral blood progenitor cell ...
-
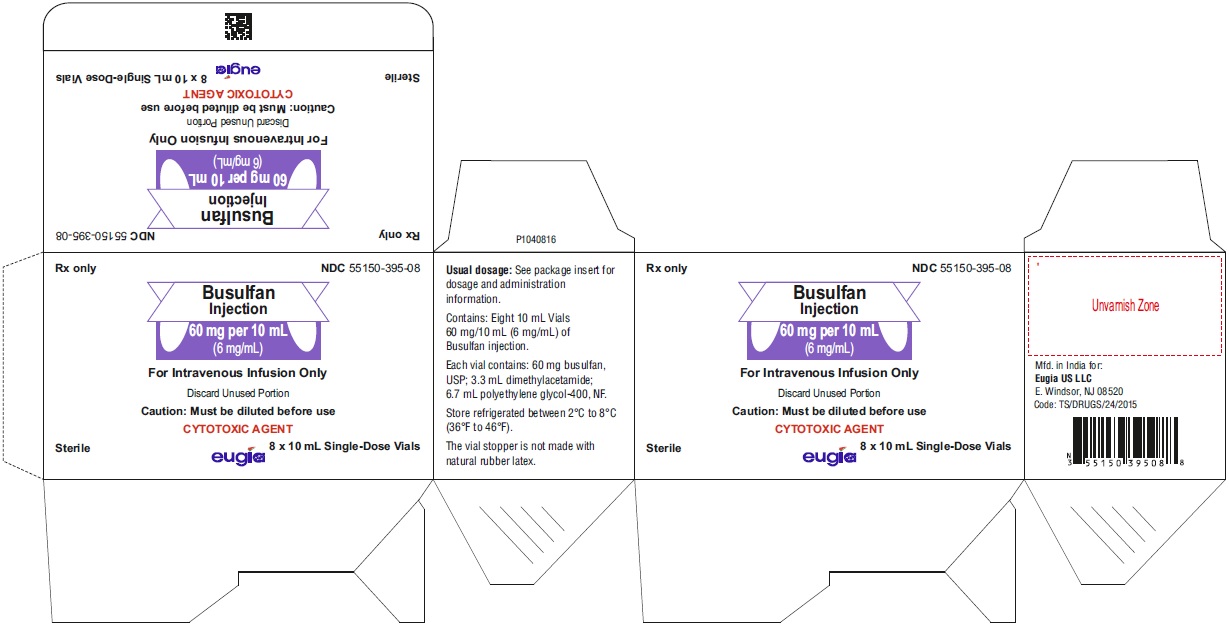
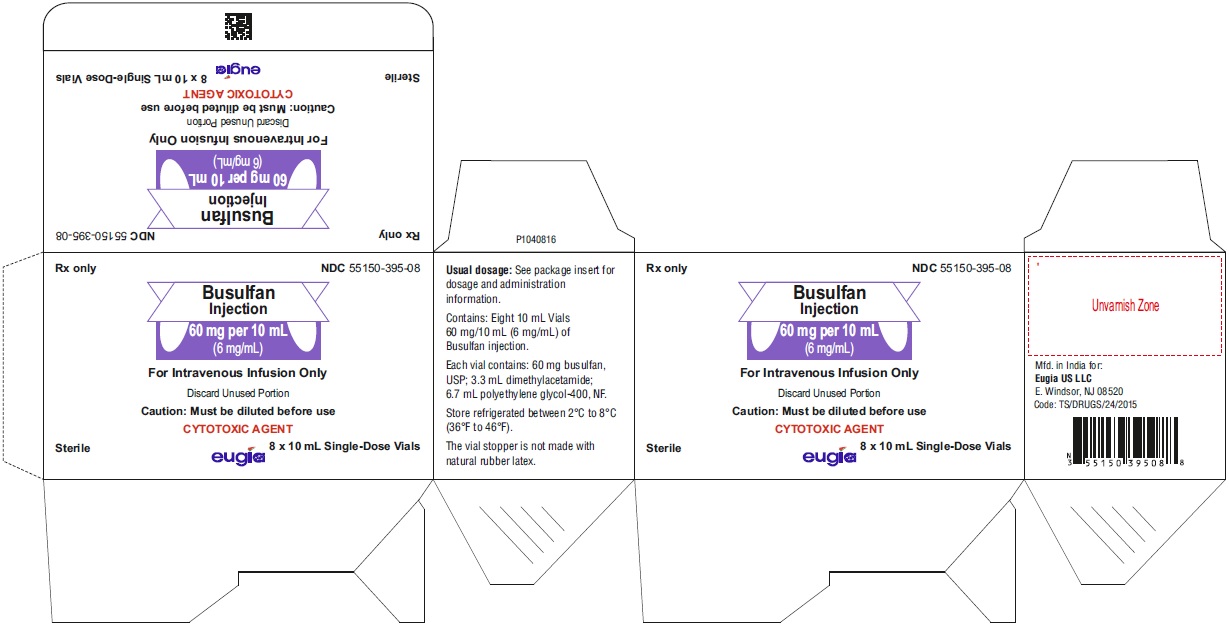
3 DOSAGE FORMS AND STRENGTHSInjection: 60 mg/10 mL (6 mg/mL) as a clear, colorless, sterile, solution in a single-dose vial for intravenous use only.
-
4 CONTRAINDICATIONSBusulfan is contraindicated in patients with a history of hypersensitivity to any of its components.
-
5 WARNINGS AND PRECAUTIONS5.1 Myelosuppression - The most frequent serious consequence of treatment with busulfan at the recommended dose and schedule is prolonged myelosuppression, occurring in all patients (100%) ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in other sections of the labeling: Myelosuppression [see Warnings and Precautions (5.1)] Seizures [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Drugs that Decrease Busulfan Clearance - Itraconazole decreases busulfan clearance by up to 25%. Metronidazole decreases the clearance of busulfan to a greater extent than does itraconazole ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Busulfan can cause fetal harm when administered to a pregnant woman based on animal data. Busulfan was teratogenic in mice, rats, and rabbits following ...
-
10 OVERDOSAGEThere is no known antidote to busulfan other than hematopoietic progenitor cell transplantation. In the absence of hematopoietic progenitor cell transplantation, the recommended dosage for ...
-
11 DESCRIPTIONBusulfan, USP is a bifunctional alkylating agent known chemically as 1,4-butanediol, dimethanesulfonate. The molecular formula of busulfan, USP is CH3SO2O(CH2)4OSO2CH3 and a molecular weight ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Busulfan is a bifunctional alkylating agent in which two labile methanesulfonate groups are attached to opposite ends of a four-carbon alkyl chain. In aqueous ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Busulfan is a mutagen and a clastogen. In in vitro tests it caused mutations in Salmonella typhimurium and Drosophila melanogaster ...
-
14 CLINICAL STUDIESDocumentation of the safety and efficacy of busulfan as a component of a conditioning regimen prior to allogeneic hematopoietic progenitor cell reconstitution is derived from two ...
-
15 REFERENCES1. OSHA Hazardous Drugs. OSHA. [Accessed on June 18, 2014 from http://www.osha.gov/SLTC/hazardousdrugs/index.html]
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Busulfan injection is packaged as a clear, colorless, sterile solution in 10 mL single-dose clear glass vials. 60 mg per 10 mL (6 mg/mL) 10 mL Single-Dose ...
-
17 PATIENT COUNSELING INFORMATIONMyelosuppression - Advise patients of the possibility of developing low blood cell counts and the need for hematopoietic progenitor cell infusion. Instruct patients to immediately report to their ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 60 mg per 10 mL (6 mg/mL) - Container LabelRx only NDC 55150-395-01 - Busulfan - Injection - 60 mg per 10 mL - (6 mg/mL) For Intravenous Infusion Only - Discard Unused Portion - Caution: Must be diluted - before use ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 60 mg/10 mL (6 mg/mL) - Container-CartonRx only NDC 55150-395-08 - Busulfan - Injection - 60 mg per 10 mL - (6 mg/mL) For Intravenous Infusion Only - Discard Unused Portion - Caution: Must be diluted before use ...
-
INGREDIENTS AND APPEARANCEProduct Information