Label: IRBESARTAN tablet
- NDC Code(s): 59746-447-05, 59746-447-30, 59746-447-90, 59746-448-05, view more
- Packager: Jubilant Cadista Pharmacuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 14, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use IRBESARTAN TABLETS safely and effectively. See full prescribing information for IRBESARTAN TABLETS. IRBESARTAN tablets, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: FETAL TOXICITY
• When pregnancy is detected, discontinue irbesartan tablets as soon as possible [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
Close
• Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
-
1 INDICATIONS AND USAGE1.1 Hypertension - Irbesartan tablets are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular (CV ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Considerations - Irbesartan tablets may be administered with other antihypertensive agents and with or without food. 2.2 Hypertension - The recommended initial dose of ...
-
3 DOSAGE FORMS AND STRENGTHSIrbesartan tablets, USP 75 mg are available as white to off-white, biconvex, oval shaped tablets, debossed with ‘447’ on one side and ‘C’ on the other. Irbesartan tablets, USP 150 mg are ...
-
4 CONTRAINDICATIONSIrbesartan is contraindicated in patients who are hypersensitive to any component of this product. Do not coadminister aliskiren with irbesartan in patients with diabetes.
-
5 WARNINGS AND PRECAUTIONS5.1 Fetal Toxicity - Irbesartan can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of ...
-
6 ADVERSE REACTIONSThe following important adverse reactions are described elsewhere in the labeling: Hypotension in Volume or Salt-Depleted Patients [see Warnings and Precautions (5.2)] Impaired Renal Function ...
-
7 DRUG INTERACTIONS7.1 Agents Increasing Serum Potassium - Coadministration of irbesartan with other drugs that raise serum potassium levels may result in hyperkalemia, sometimes severe. Monitor serum potassium in ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Irbesartan can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third ...
-
10 OVERDOSAGENo data are available in regard to overdosage in humans. However, daily doses of 900 mg for 8 weeks were well-tolerated. The most likely manifestations of overdosage are expected to be hypotension ...
-
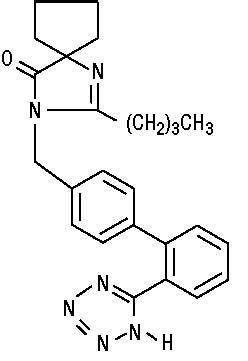
11 DESCRIPTIONIrbesartan, USP is an angiotensin II receptor (AT1 subtype) antagonist. Irbesartan, USP is a non-peptide compound, chemically described as a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Angiotensin II is a potent vasoconstrictor formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No evidence of carcinogenicity was observed when irbesartan was administered at dosages of up to 500/1000 mg/kg/day (males/females ...
-
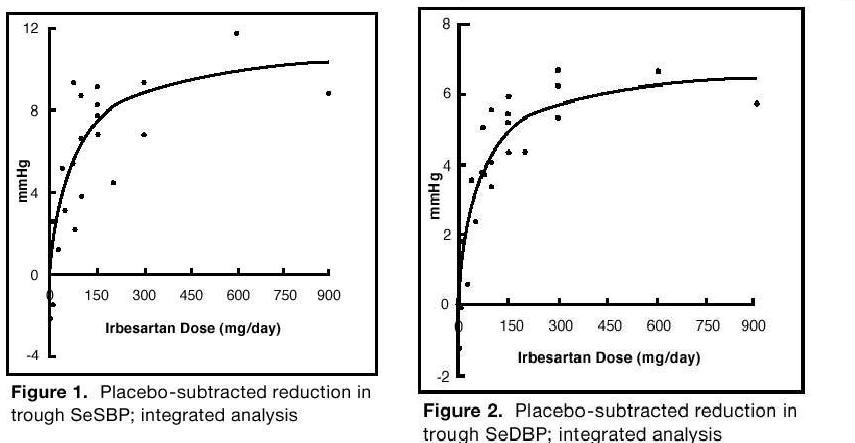
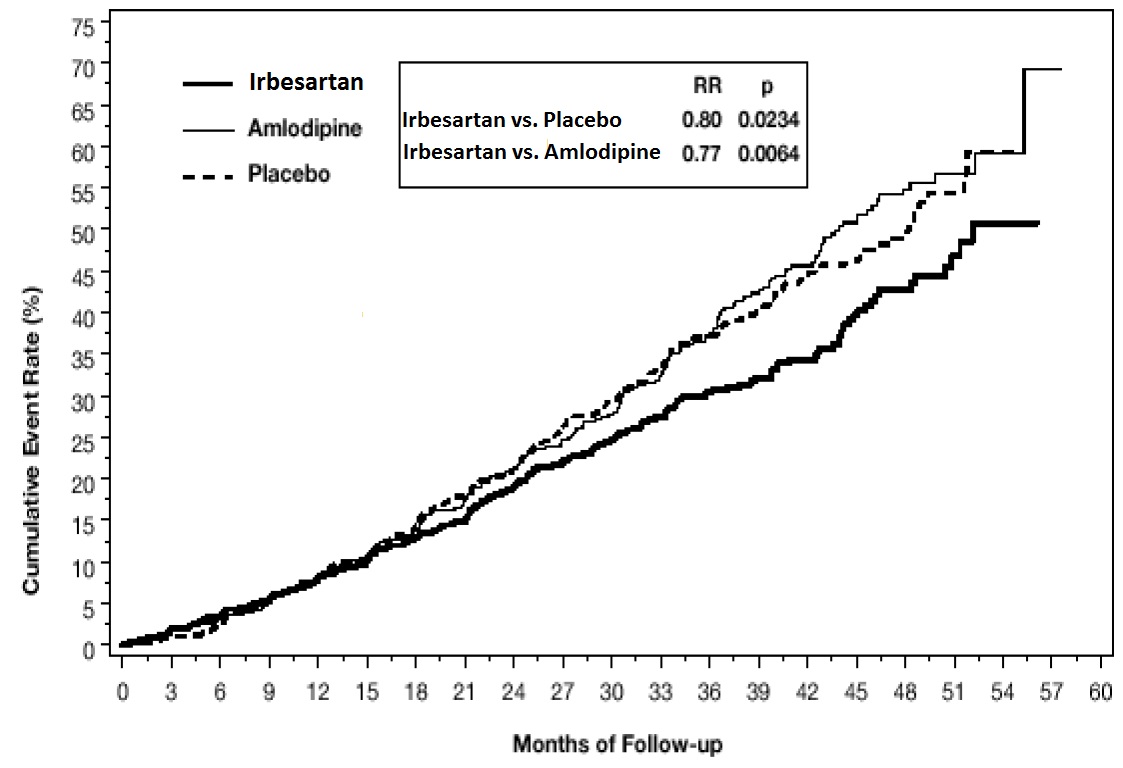
14 CLINICAL STUDIES14.1 Hypertension - The antihypertensive effects of irbesartan were examined in 7 placebo-controlled 8- to 12-week trials in patients with baseline diastolic blood pressures of 95 to 110 mmHg ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGIrbesartan Tablets, USP 75 mg are available as white to off-white, biconvex, oval shaped tablets, debossed with ‘447’ on one side and ‘C’ on the other. Bottle of 30’s (child Resistant ...
-
17 PATIENT COUNSELING INFORMATIONPregnancy - Advise female patients of childbearing age about the consequences of exposure to irbesartan during pregnancy. Discuss treatment options with women planning to become pregnant ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 59746-447-30 - Irbesartan Tablets, USP - 75 mg - 30 Tablets Rx Only - CADISTATM - NDC 59746-448-30 - Irbesartan Tablets, USP - 150 mg - 30 Tablets Rx Only ...
-
INGREDIENTS AND APPEARANCEProduct Information