Label: TOBRAMYCIN AND DEXAMETHASONE suspension/ drops
- NDC Code(s): 61314-647-05, 61314-647-10, 61314-647-25
- Packager: Sandoz Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated April 7, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
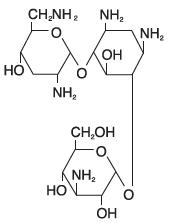
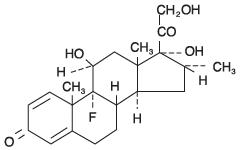
DESCRIPTIONDESCRIPTION: Tobramycin and dexamethasone ophthalmic suspension is a sterile, multiple dose antibiotic and steroid combination for topical ophthalmic use. The chemical structures for tobramycin ...
-
CLINICAL PHARMACOLOGYCLINICAL PHARMACOLOGY: Corticoids suppress the inflammatory response to a variety of agents and they probably delay or slow healing. Since corticoids may inhibit the body's defense mechanism ...
-
INDICATIONS & USAGEINDICATIONS AND USAGE: Tobramycin and dexamethasone ophthalmic suspension is indicated for steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where ...
-
CONTRAINDICATIONSCONTRAINDICATIONS: Epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, and many other viral diseases of the cornea and conjunctiva. Mycobacterial infection of the eye ...
-
WARNINGSWARNINGS: FOR EYE USE ONLY. NOT FOR INJECTION INTO THE EYE. Sensitivity to topically applied aminoglycosides may occur in some patients. Severity of hypersensitivity reactions may vary from local ...
-
PRECAUTIONS:General: The possibility of fungal infections of the cornea should be considered after long-term steroid dosing. As with other antibiotic preparations, prolonged use may result in overgrowth ...
-
NURSING MOTHERSNursing Mothers: Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It ...
-
PEDIATRIC USEPediatric Use: Safety and effectiveness in pediatric patients below the age of 2 years have not been established.
-
GERIATRIC USEGeriatric Use: No overall differences in safety or effectiveness have been observed between elderly and younger patients.
-
ADVERSE REACTIONSADVERSE REACTIONS: Adverse reactions have occurred with steroid/anti-infective combination drugs which can be attributed to the steroid component, the anti-infective component, or the combination ...
-
DOSAGE & ADMINISTRATIONDOSAGE AND ADMINISTRATION: One or two drops instilled into the conjunctival sac(s) every four to six hours. During the initial 24 to 48 hours, the dosage may be increased to one or two drops every ...
-
HOW SUPPLIEDHOW SUPPLIED: Sterile ophthalmic suspension in 2.5 mL (NDC 61314-647-25), 5 mL (NDC 61314-647-05) and 10 mL (NDC 61314-647-10) dispensers. STORAGE: Store at 8°C to 27°C (46°F to 80°F). Store ...
-
PRINCIPAL DISPLAY PANELNDC 61314-647-25 Rx Only - Tobramycin - 0.3% and - Dexamethasone - 0.1% Ophthalmic - Suspension, USP - FOR EYE USE ONLY - STERILE - 2.5 mL - SANDOZ
-
INGREDIENTS AND APPEARANCEProduct Information