Label: CLONIDINE TRANSDERMAL SYSTEM USP, 0.1 MG/DAY- clonidine transdermal system patch
CLONIDINE TRANSDERMAL SYSTEM USP, 0.2 MG...view full title
CLONIDINE TRANSDERMAL SYSTEM USP, 0.2 MG...
- NDC Code(s): 52817-610-04, 52817-611-04, 52817-612-04
- Packager: TruPharma, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated April 17, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONProgrammed delivery in vivo of 0.1, 0.2, or 0.3 mg clonidine per day, for one week. Rx only - Prescribing Information
-
DESCRIPTION
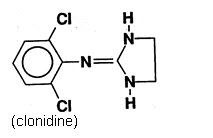
Clonidine Transdermal System, USP provides continuous systemic delivery of clonidine for 7 days at an approximately constant rate. Clonidine is a centrally acting alpha‑agonist hypotensive agent ...
-
CLINICAL PHARMACOLOGY
Clonidine stimulates alpha‑adrenoreceptors in the brain stem. This action results in reduced sympathetic outflow from the central nervous system and in decreases in peripheral resistance, renal ...
-
INDICATIONS AND USAGE
Clonidine transdermal system is indicated in the treatment of hypertension. It may be employed alone or concomitantly with other antihypertensive agents.
-
CONTRAINDICATIONS
Clonidine transdermal system should not be used in patients with known hypersensitivity to clonidine or to any other component of the transdermal system.
-
WARNINGS
Withdrawal - Patients should be instructed not to discontinue therapy without consulting their physician. Sudden cessation of clonidine treatment has, in some cases, resulted in symptoms such ...
-
PRECAUTIONS
General - In patients who have developed localized contact sensitization to clonidine transdermal system, continuation of clonidine transdermal system or substitution of oral clonidine ...
-
ADVERSE REACTIONS
Clinical trial experience with clonidine transdermal system - Most systemic adverse effects during clonidine transdermal system therapy have been mild and have tended to diminish with ...
-
OVERDOSAGE
Hypertension may develop early and may be followed by hypotension, bradycardia, respiratory depression, hypothermia, drowsiness, decreased or absent reflexes, weakness, irritability and miosis ...
-
DOSAGE AND ADMINISTRATION
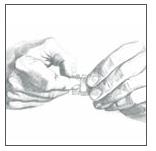
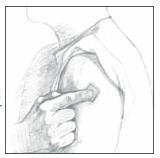
Apply Clonidine Transdermal System, USP once every 7 days to a hairless area of intact skin on the upper outer arm or chest. Each new application of Clonidine Transdermal System, USP should be on ...
-
HOW SUPPLIED
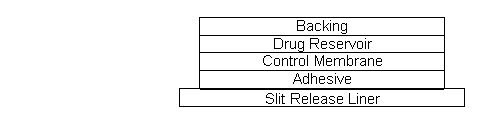
Clonidine Transdermal System USP, 0.1 mg/day, Clonidine Transdermal System USP, 0.2 mg/day, and Clonidine Transdermal System USP, 0.3 mg/day are supplied as 4 pouched systems and 4 adhesive ...
-
STORAGE AND HANDLING
Store at 25°C (77°F); excursions permitted to 15°- 30°C (59°- 86°F) [see USP Controlled Room Temperature]. Distributed by: TruPharma LLC, Tampa FL 33609, USA - Address medical inquiries to ...
-
PATIENT INSTRUCTIONS
Clonidine Transdermal System, USP - (Read the following instructions carefully before using this medication. If you have any questions, please consult with your doctor.) General ...
-
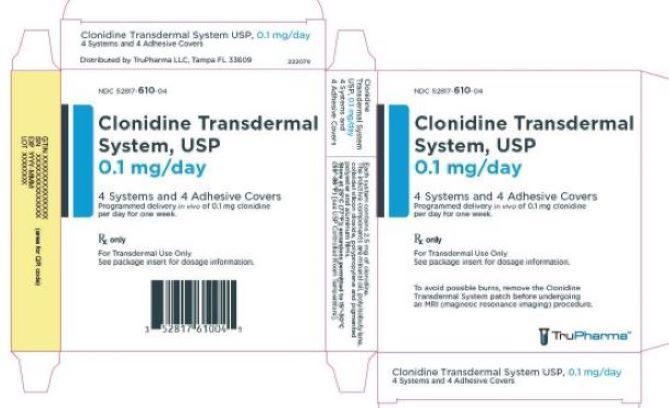
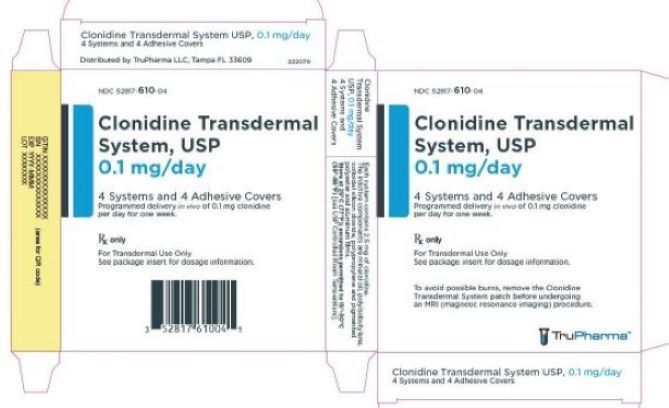
PRINCIPAL DISPLAY PANEL - 0.1 mg CartonNDC 52817-610-04 - Clonidine Transdermal - System, USP - 0.1 mg/day - 4 Systems and 4 Adhesive Covers - Programmed delivery in vivo of 0.1 mg clonidine - per day for one week. Rx only - For ...
-
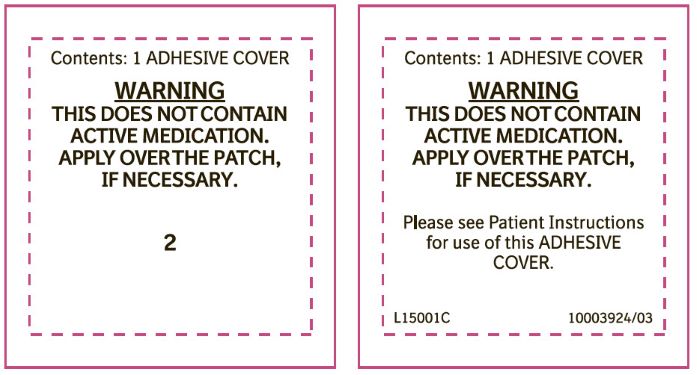
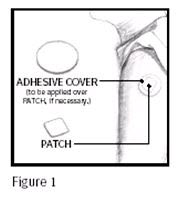
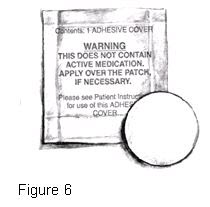
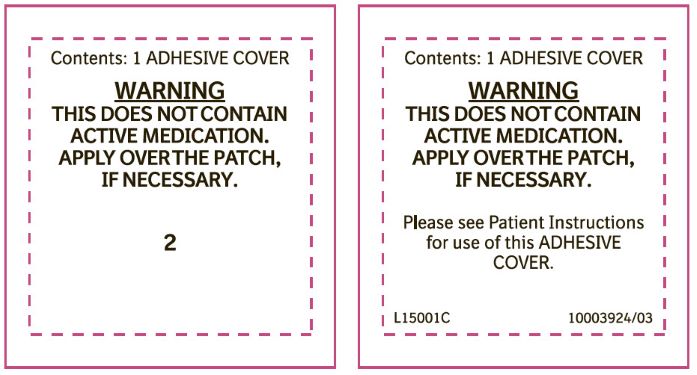
PRINCIPAL DISPLAY PANEL - 0.1 mg Adhesive CoverContents: 1 ADHESIVE COVER - WARNING - THIS DOES NOT CONTAIN - ACTIVE MEDICATION. APPLY OVER THE PATCH, IF NECESSARY. 1
-
PRINCIPAL DISPLAY PANEL - 0.1 mg PouchClonidine - Transdermal System - USP - 01. mg/day - Programmed delivery in vivo of 0.1 - mg clonidine per day for one week. To avoid possible burns, remove the - clinidine transdermal system ...
-
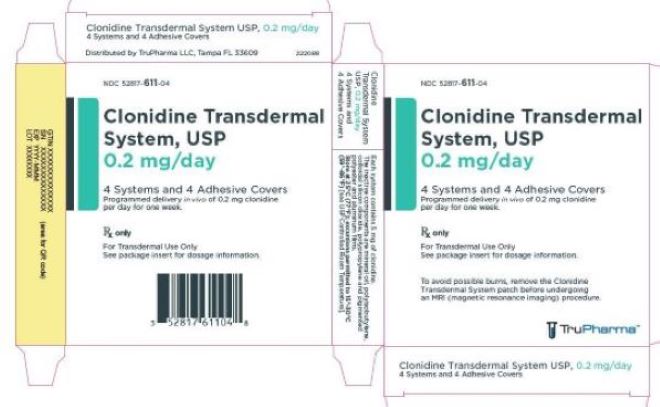
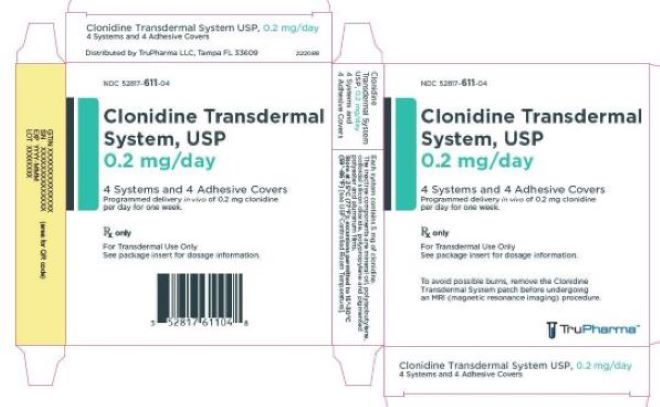
PRINCIPAL DISPLAY PANEL - 0.2 mg CartonNDC 52817-611-04 - Clonidine Transdermal - System, USP - 0.2 mg/day - 4 Systems and 4 Adhesive Covers - Programmed delivery in vivo of 0.2 mg clonidine - per day for one week. Rx only - For ...
-
PRINCIPAL DISPLAY PANEL - 0.2 mg Adhesive CoverContents: 1 ADHESIVE COVER - WARNING - THIS DOES NOT CONTAIN - ACTIVE MEDICATION. APPLY OVER THE PATCH, IF NECESSARY. 2
-
PRINCIPAL DISPLAY PANEL - 0.2 mg PouchClonidine - Transdermal System - USP - 0.2 mg/day - Programmed delivery in vivo of 0.2 - mg clonidine per day for one week. To avoid possible burns, remove the - clonidine transdermal system ...
-
PRINCIPAL DISPLAY PANEL carton - 0.3 mgNDC 52817-612-04 - Clonidine Transdermal - System, USP - 0.3 mg/day - 4 Systems and 4 Adhesive Covers - Programmed delivery in vivo of 0.3 mg clonidine - per day for one week. Rx only - For ...
-
PRINCIPAL DISPLAY PANEL - 0.3 mg Adhesive CoverContents: 1 ADHESIVE COVER - WARNING - THIS DOES NOT CONTAIN - ACTIVE MEDICATION. APPLY OVER THE PATCH, IF NECESSARY. 3
-
PRINCIPAL DISPLAY PANEL - 0.3 mg PouchClonidine - Transdermal System - USP - 0.3 mg/day - Programmed delivery in vivo of 0.3 mg clonidine - per day for one week. To avoid possible burns, remove the - clonidine transdermal system ...
-
INGREDIENTS AND APPEARANCEProduct Information
View Labeling Archives for this drug
CLONIDINE TRANSDERMAL SYSTEM USP, 0.1 MG/DAY- clonidine transdermal system patch
CLONIDINE TRANSDERMAL SYSTEM USP, 0.2 MG...view full title
CLONIDINE TRANSDERMAL SYSTEM USP, 0.2 MG...
Number of versions: 3
RxNorm
CLONIDINE TRANSDERMAL SYSTEM USP, 0.1 MG/DAY- clonidine transdermal system patch
CLONIDINE TRANSDERMAL SYSTEM USP, 0.2 MG...view full title
CLONIDINE TRANSDERMAL SYSTEM USP, 0.2 MG...
Get Label RSS Feed for this Drug
CLONIDINE TRANSDERMAL SYSTEM USP, 0.1 MG/DAY- clonidine transdermal system patch
CLONIDINE TRANSDERMAL SYSTEM USP, 0.2 MG...view full title
CLONIDINE TRANSDERMAL SYSTEM USP, 0.2 MG...
NDC Codes
CLONIDINE TRANSDERMAL SYSTEM USP, 0.1 MG/DAY- clonidine transdermal system patch
CLONIDINE TRANSDERMAL SYSTEM USP, 0.2 MG...view full title
CLONIDINE TRANSDERMAL SYSTEM USP, 0.2 MG...