Label: ZOLMITRIPTAN spray, metered
- NDC Code(s): 69238-2351-6, 69238-2352-6
- Packager: Amneal Pharmaceuticals NY LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated April 30, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZOLMITRIPTAN NASAL SPRAY safely and effectively. See full prescribing information for ZOLMITRIPTAN NASAL SPRAY. ZOLMITRIPTAN ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZOLMITRIPTAN NASAL SPRAY is indicated for the acute treatment of migraine with or without aura in adults and pediatric patients 12 years of age and older. Limitations of Use - Only use ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The recommended starting dose for ZOLMITRIPTAN NASAL SPRAY in adult and pediatric patients 12 years of age and older is 2.5 mg. As the individual response to ZOLMITRIPTAN ...
-
3 DOSAGE FORMS AND STRENGTHSNasal Spray 2.5 mg and 5 mg.
-
4 CONTRAINDICATIONSZolmitriptan is contraindicated in patients with: Ischemic coronary artery disease (angina pectoris, history of myocardial infarction, or documented silent ischemia), other significant underlying ...
-
5 WARNINGS AND PRECAUTIONS5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal's Angina - Zolmitriptan is contraindicated in patients with ischemic or vasospastic coronary artery disease (CAD). There have been ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in other sections of labeling: Myocardial Ischemia, Myocardial Infarction, and Prinzmetal's Angina [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Ergot-containing drugs - Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Because these effects may be additive, use of ergotamine-containing or ergot-type ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with the use of zolmitriptan in pregnant women. In reproductive toxicity studies in rats and ...
-
10 OVERDOSAGEThere is no experience with acute overdose. Clinical study subjects receiving single 50 mg oral doses of zolmitriptan commonly experienced sedation. The elimination half-life of zolmitriptan is 3 ...
-
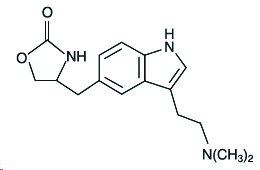
11 DESCRIPTIONZOLMITRIPTAN NASAL SPRAY contains zolmitriptan, which is a selective 5-hydroxytryptamine 1B/1D (5-HT1B/1D) receptor agonist. Zolmitriptan is chemically designated as (S)-4-[[3-[2-(dimethylamino ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Zolmitriptan binds with high affinity to human recombinant 5-HT1D and 5-HT1B receptors, and moderate affinity for 5-HT1A receptors. The N-desmethyl metabolite also has ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Zolmitriptan was administered to mice and rats at doses up to 400 mg/kg/day. Mice were dosed for 85 weeks (males) and ...
-
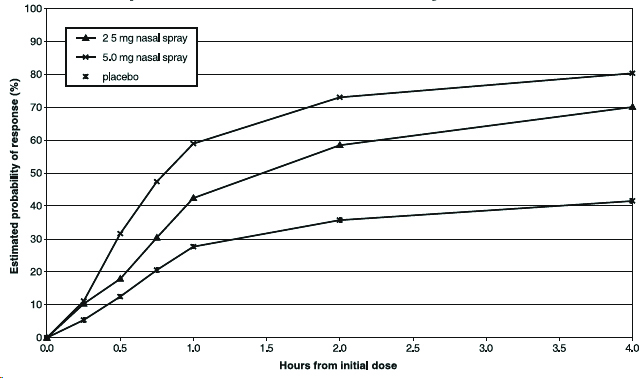
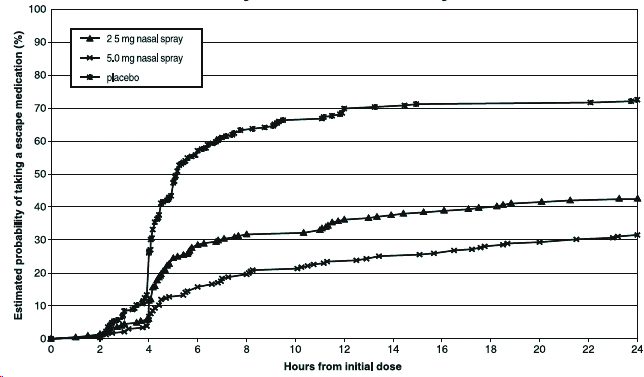
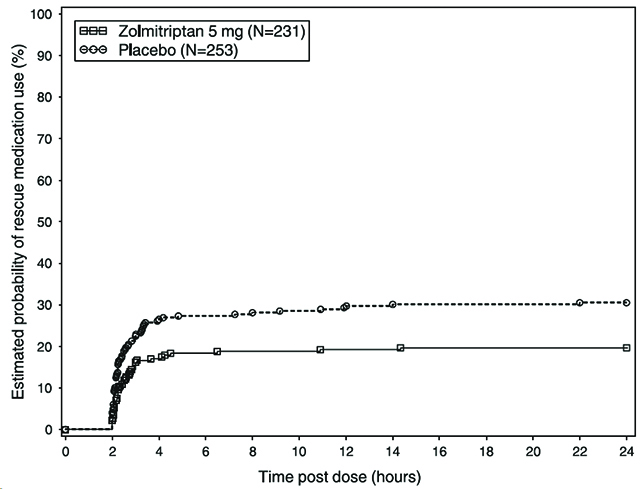
14 CLINICAL STUDIES14.1 Adults - The efficacy of zolmitriptan nasal spray 2.5 mg and 5 mg in the acute treatment of migraine headache with or without aura in adults was demonstrated in Study 1, a randomized ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGThe ZOLMITRIPTAN NASAL SPRAY device is a blue-colored plastic device with a gray protection cap, labeled to indicate the nominal dose. Each ZOLMITRIPTAN NASAL SPRAY device administers a single ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Risk of Myocardial Ischemia and/or Infarction, Prinzmetal's angina, Other Vasospasm-related Events, and ...
-
Patient InformationZOLMITRIPTAN (ZOLE-mi-TRIP-tan) NASALSPRAY - Please read this information before you start taking ZOLMITRIPTAN NASAL SPRAY and each time you renew your prescription just in case anything has ...
-
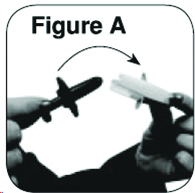
Instructions for UseZOLMITRIPTAN (ZOLE-mi-TRIP-tan) Nasal Spray - Important: For use in your nose only. Do not spray in your eyes. Note: There is only 1 dose in the nasal sprayer. Do not try to prime the ...
-
PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information