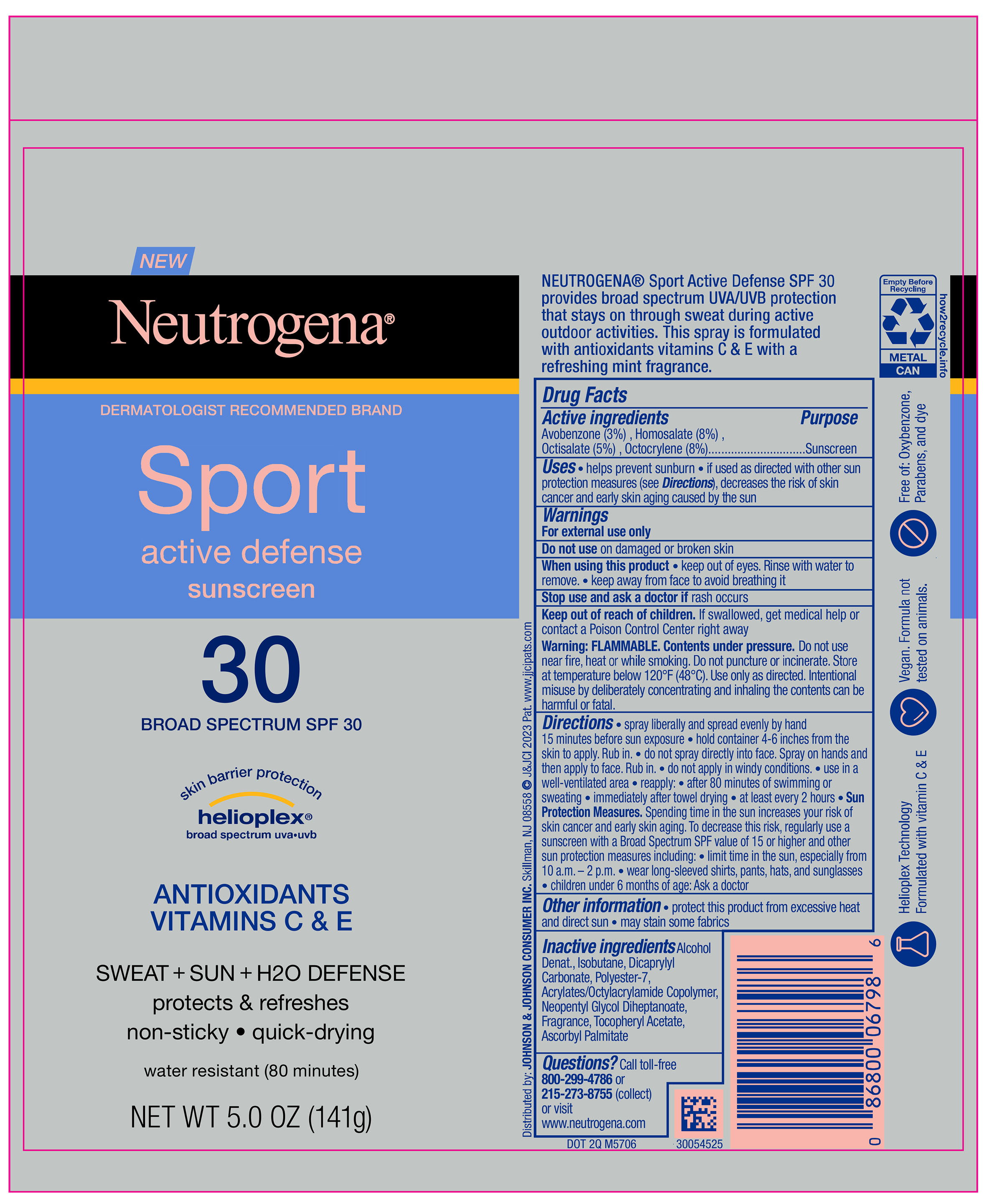
Label: NEUTROGENA SPORT ACTIVE DEFENSE SPF 30- avobenzone, homosalate, octisalate, octocrylene spray
- NDC Code(s): 69968-0824-5
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
-
Directions
• spray liberally and spread evenly by hand 15 minutes before sun exposure
• hold container 4-6 inches from the skin to apply. Rub in.
• do not spray directly into face. Spray on hands and then apply to face. Rub in.
• do not apply in windy conditions.
• use in a well-ventilated area
• reapply:
• after 80 minutes of swimming or sweating
• immediately after towel drying
• at least every 2 hours
• Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
• children under 6 months of age: Ask a doctor
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 141 g Can Label
NEW
Neutrogena ®
DERMATOLOGIST RECOMMENDED BRAND
Sport
active defense
sunscreen
30
BROAD SPECTRUM SPF 30
skin barrier protection
helioplex ®
broad spectrum uva●uvb
ANTIOXIDANTS
VITAMINS C & E
SWEAT + SUN + H2O DEFENSE
protects & refreshes
non-sticky ● quick-drying
water resistant (80 minutes)
NET WT 5.0 OZ (141 g)
-
INGREDIENTS AND APPEARANCE
NEUTROGENA SPORT ACTIVE DEFENSE SPF 30
avobenzone, homosalate, octisalate, octocrylene sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0824 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 80 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 80 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength ASCORBYL PALMITATE (UNII: QN83US2B0N) ALCOHOL (UNII: 3K9958V90M) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) POLYESTER-7 (UNII: 0841698D2F) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) ACRYLATE/ISOBUTYL METHACRYLATE/N-TERT-OCTYLACRYLAMIDE COPOLYMER (75000 MW) (UNII: JU3XHR8VWK) ISOBUTANE (UNII: BXR49TP611) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0824-5 141 g in 1 CAN; Type 0: Not a Combination Product 12/04/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/04/2023 Labeler - Kenvue Brands LLC (118772437)