Label: POLYETHYLENE GLYCOL 3350 powder, for solution
- NDC Code(s): 70000-0699-2
- Packager: LEADER/ CARDINAL HEALTH 110, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Use
- Warnings
- DO NOT USE
- Ask a doctor before use if you have
- ASK DOCTOR/PHARMACIST
- WHEN USING
- Stop use and ask a doctor if
- PREGNANCY OR BREAST FEEDING
- Keep out of reach of children.
-
Directions
- do not take more than directed unless advised by your doctor
- adults and children 17 years of age and older:
- use once a day
- stir and dissolve one packet of powder (17 g) in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- do not combine with starch-based thickeners used for difficulty swallowing
- ensure that the powder is fully dissolved before drinking
- do not drink if there are any clumps
- do not use more than 7 days
- children 16 years of age or under: ask a doctor
- Other information
- Inactive ingredients
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 17 g Pouch Label
LEADERTM
NDC 70000-0699-1
Grit-Free | Unflavored Powder Packet
Polyethylene
Glycol 3350
Powder for Oral Solution
Osmotic Laxative
Relieves Occasional
Constipation/Irregularity
Softens Stool
Sugar-Free
Dissolves in any Beverage
COMPARE TO
MiraLAX®
active ingredient*
100% Money
Back Guarantee
1 DOSE
NET WT 0.5 OZ (17g)

-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 17 g Pouch Carton Label
LEADERTM
NDC 70000-0699-2
Grit-Free | Unflavored Powder Packets
Polyethylene Glycol 3350
Powder for Oral Solution | Osmotic Laxative
Relieves Occasional Constipation/Irregularity
Softens Stool
Sugar-Free
Dissolves in Any Beverage
COMPARE TO
MiraLAX®
active ingredient*
100% Money
Back Guarantee
10 PRE-MEASURED, SINGLE DOSES 10 PACKETS
10 ONCE-DAILY DOSES NET WT 0.5 OZ (17g) EACH
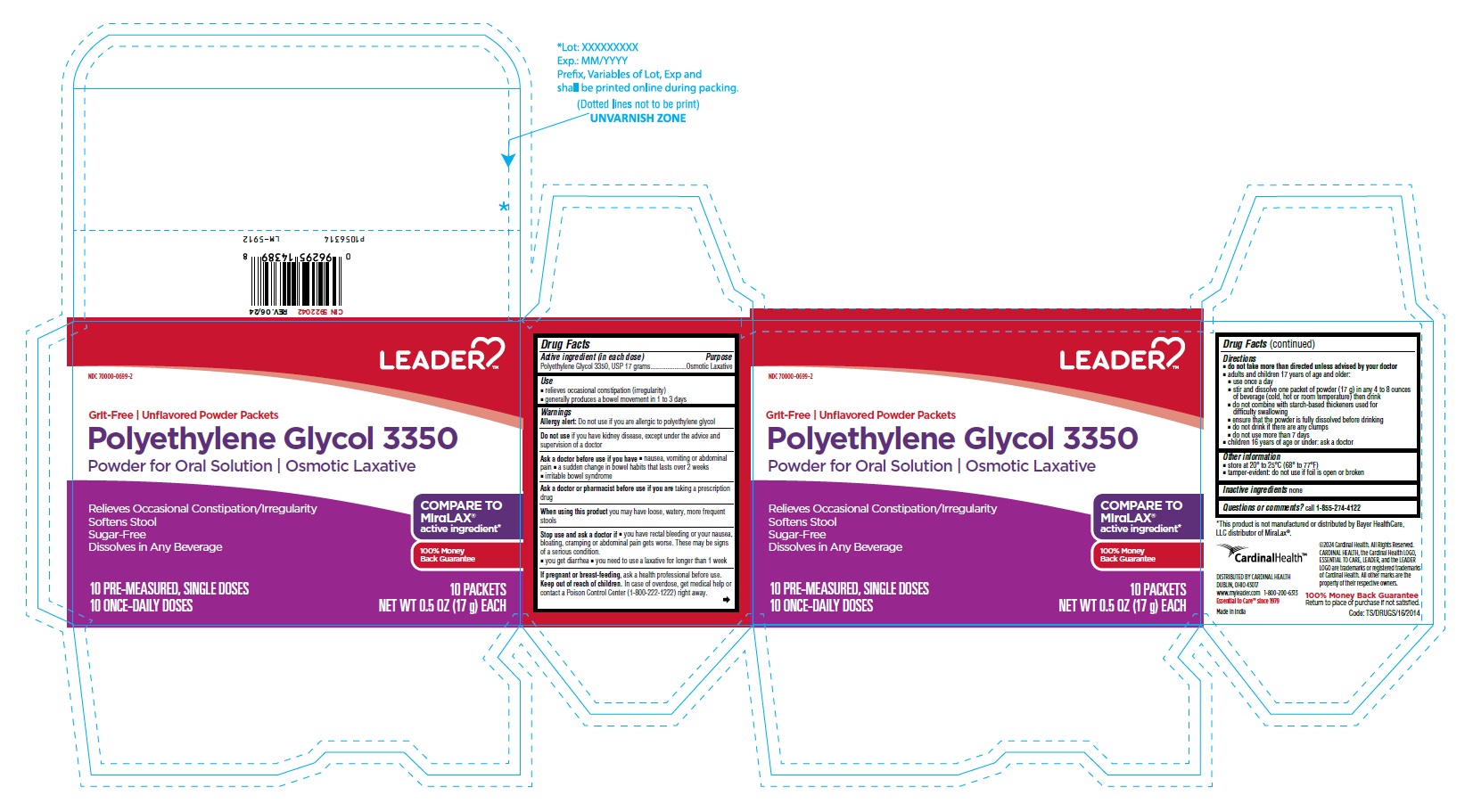
-
INGREDIENTS AND APPEARANCE
POLYETHYLENE GLYCOL 3350
polyethylene glycol 3350 powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70000-0699 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 17 g in 17 g Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70000-0699-2 10 in 1 CARTON 11/08/2024 1 17 g in 1 PACKET; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209017 11/08/2024 Labeler - LEADER/ CARDINAL HEALTH 110, INC. (063997360) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations APL HEALTHCARE LIMITED 650844777 ANALYSIS(70000-0699) , MANUFACTURE(70000-0699) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 918917642 ANALYSIS(70000-0699) , MANUFACTURE(70000-0699)


