Label: ISOPROPYL ALCOHOL liquid
- NDC Code(s): 82749-004-16
- Packager: Epic Medical Supply Corp.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredient
-
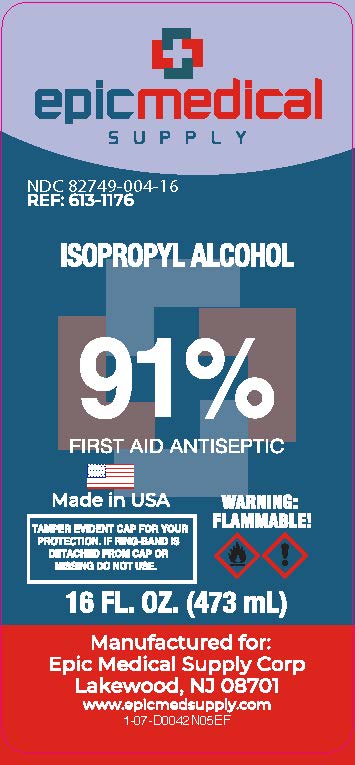
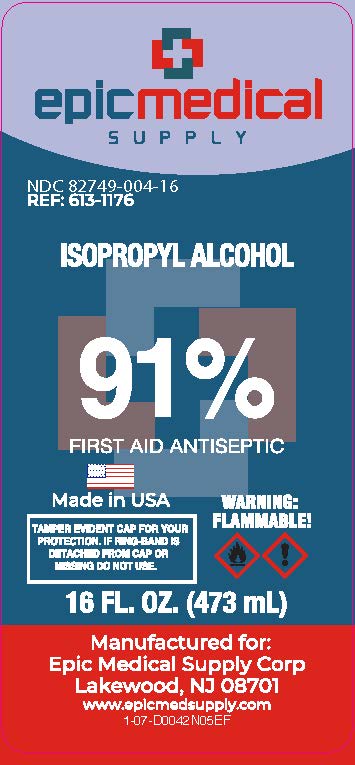
PRINCIPAL DISPLAY PANEL
Epic Medical Supply
91% by Volume
ISOPROPYL ALCOHOL
First Aid Antiseptic
Square bottle uses less plastic than a similarly sized round bottle
Recyclable (If available in your area)
TAMPER EVIDENT CAP FOR YOUR PROTECTION.
IF RING-BAND IS DETACHED FROM CAP OR MISSING DO NOT USE.
Warning
Flammable
Manufactured for Epic Medical Supply Corp
Lakewood New Jersey 08701
www.epicmedsupply.com
16 FL. OZ. (473 mL)

-
INGREDIENTS AND APPEARANCE
ISOPROPYL ALCOHOL
isopropyl alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82749-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 91 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82749-004-16 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/13/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 03/13/2023 Labeler - Epic Medical Supply Corp. (101423894)