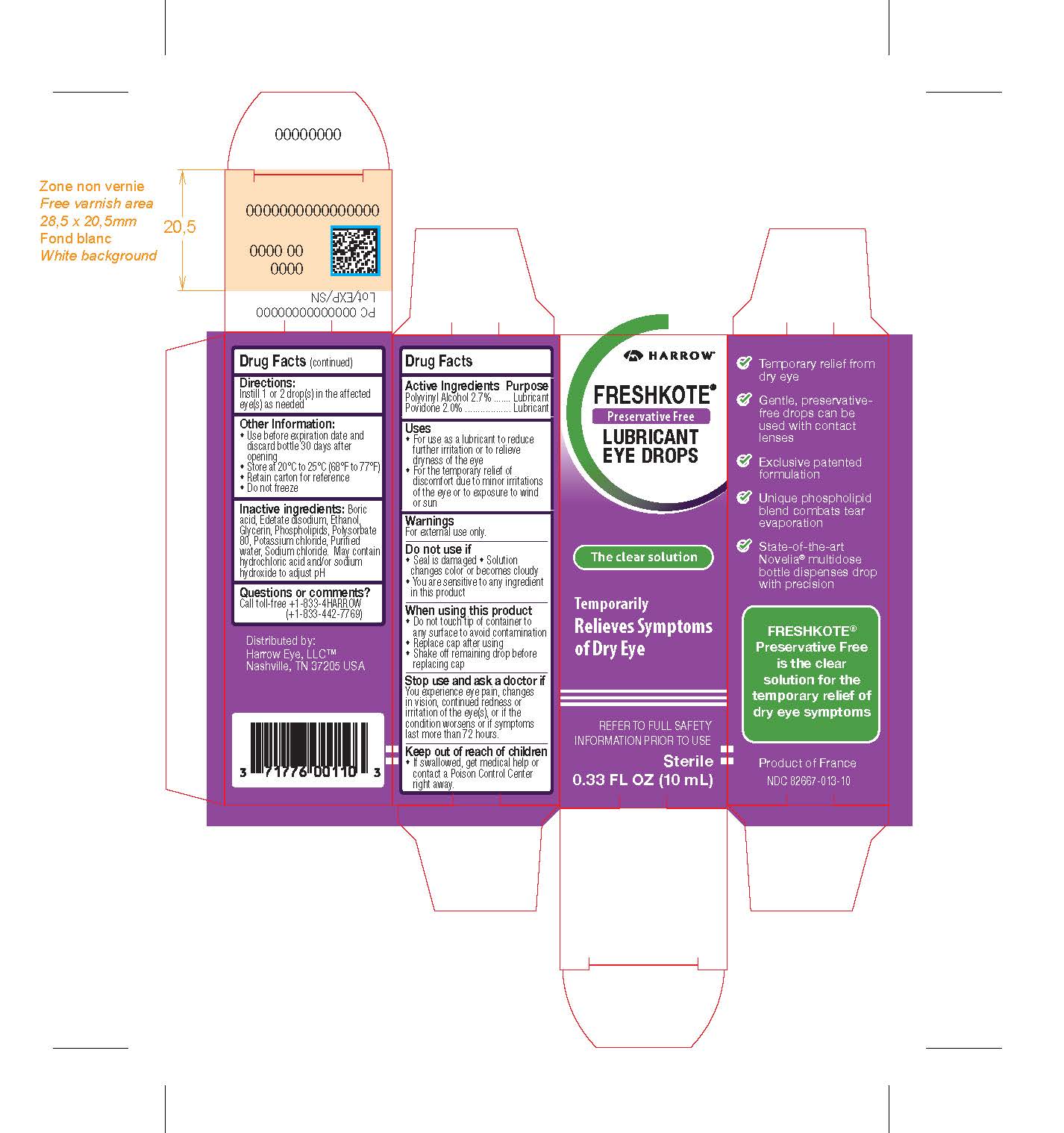
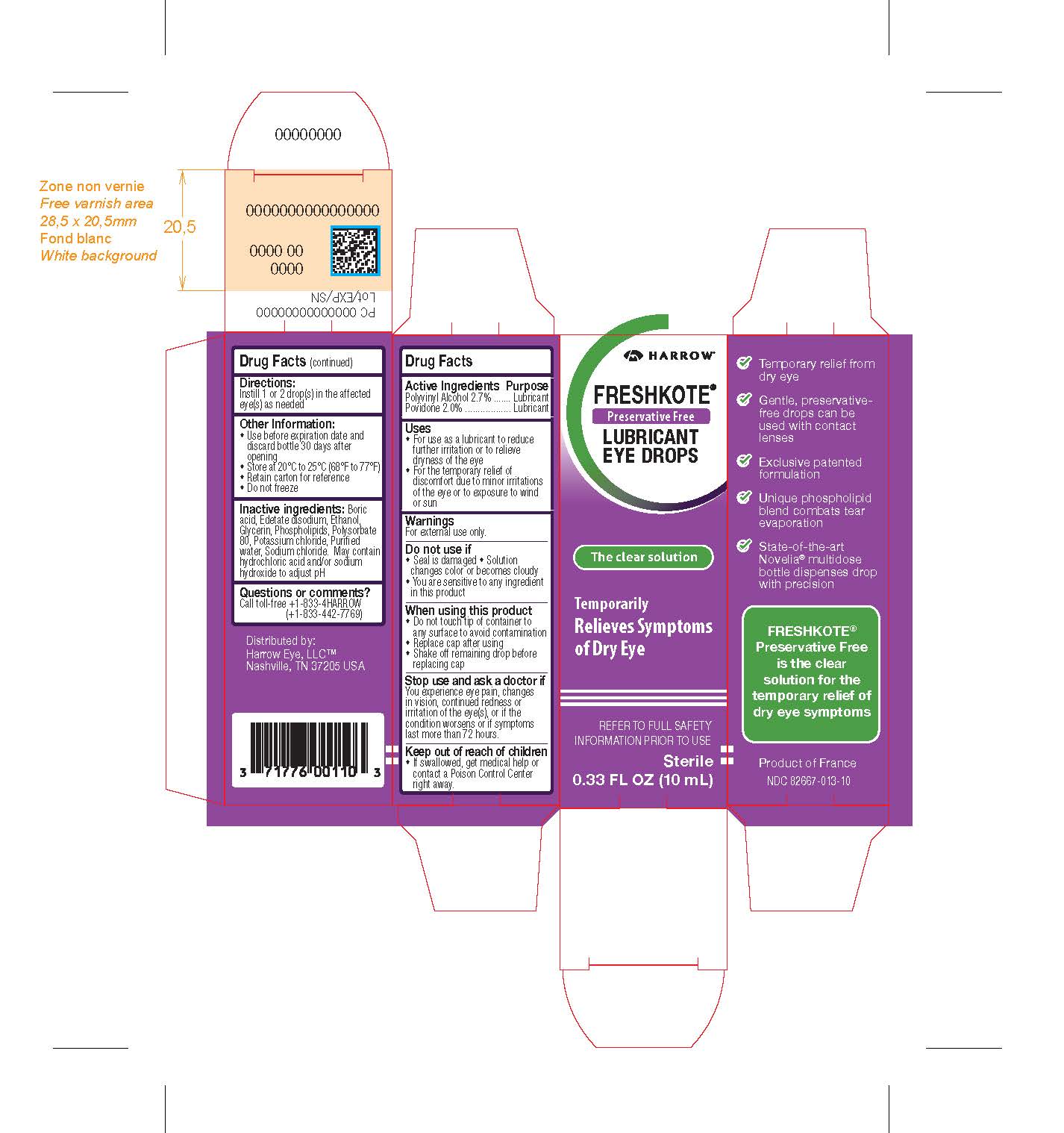
Label: FRESHKOTE PRESERVATIVE FREE LUBRICANT EYE DROPS- povidone, polyvinyl alcohol solution solution/ drops
- NDC Code(s): 82667-013-10
- Packager: Harrow Eye, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- PURPOSE
-
PRINCIPAL DISPLAY PANEL
HARROW
FRESHKOTE®
Preservative Free
LUBRICANT EYE DROPS
The clear solution
Temporarily Relieves Symptoms of Dry Eye
REFER TO FULL SAFETY INFORMATION PRIOR TO USE
Sterile
0.33 FL OZ (10mL)
Temporary relief from dry eye
Gentle, preservative-free drops can be used with contact lenses
Exclusive patented formulation
Unique phospholipid blend combats tear evaporation
State-of-the-art Novelia® multidose bottle dispenses drop with precision
FRESHKOTE®
Preservative Free
is the clear
solution for the
temporary relief of
dry eye symptoms
Product of France
NDC 82667-013-10
-
INGREDIENTS AND APPEARANCE
FRESHKOTE PRESERVATIVE FREE LUBRICANT EYE DROPS
povidone, polyvinyl alcohol solution solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82667-013 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) (POLYVINYL ALCOHOL, UNSPECIFIED - UNII:532B59J990) POLYVINYL ALCOHOL, UNSPECIFIED 27 g in 1000 mL POVIDONE (UNII: FZ989GH94E) (POVIDONE - UNII:FZ989GH94E) POVIDONE 20 g in 1000 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) EDETATE DISODIUM (UNII: 7FLD91C86K) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POTASSIUM CHLORIDE (UNII: 660YQ98I10) BORIC ACID (UNII: R57ZHV85D4) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82667-013-10 1 in 1 BOX 09/15/2023 1 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 09/15/2023 Labeler - Harrow Eye, LLC (118526951)