Label: FLUDEOXYGLUCOSE F 18- fludeoxyglucose f-18 injection, solution
- NDC Code(s): 76376-618-30
- Packager: Hamamatsu/Queen’s PET Imaging Center, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 11, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
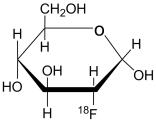
Fludeoxyglucose F 18 Injection, USP is a positron emitting radiopharmaceutical containing no-carrier added radioactive 2-deoxy-2-[18F]fluoro-D-g1ucose, which is used for diagnostic purposes ...
-
CLINICAL PHARMACOLOGY
Mechanism of Action - Fludeoxyglucose F 18 is a glucose analog that concentrates in cells that rely upon glucose as an energy source, or in cells whose dependence on glucose increases under ...
-
CLINICAL TRIALS
Oncology:1 The efficacy of Fludeoxyglucose F 18 Injection in positron emission tomography cancer imaging was demonstrated in 16 independent literature reports. These studies prospectively ...
-
INDICATIONS AND USAGE
Fludeoxyglucose F 18 Injection, USP is indicated in positron emission tomography (PET) imaging for assessment of abnormal glucose metabolism to assist in the evaluation of malignancy in ...
-
CONTRAINDICATIONS
None known
-
WARNINGS
None known
-
PRECAUTIONS
General - Use in patients with diabetes mellitus or hyperglycemia has not been well studied. It is recommended that patients be normoglycemic when undergoing PET imaging with Fludeoxyglucose F ...
-
ADVERSE REACTIONS
The Fludeoxyglucose F 18 Injection safety database for epilepsy included of 374 patients. Of these, 245 were male and 105 were female. For 24 patients, gender was not specified. The mean age was ...
-
DOSAGE AND ADMINISTRATION
The recommended dose of Fludeoxyglucose F 18 Injection for an adult (70 kg) is 185-370 MBq (5-10 mCi), as an intravenous injection for studies of malignancy, cardiology, and epilepsy. In general ...
-
OVERDOSAGE
Overdoses of Fludeoxyglucose F 18 Injection have not been reported. See Radiation Dosimetry section for related information.
-
DRUG HANDLING
Fludeoxyglucose F 18 Injection, USP, like other parenteral drugs, should be inspected visually for particulate matter and discoloration before administration, whenever solution and ...
-
RADIATION DOSIMETRY
The estimated human absorbed radiation doses (rem/mCi) to a 1-year old (9.8 kg), 5-year old (19 kg), 10-year old (32 kg), 15-year old (57 kg), and adult (70 kg) from intravenous administration of ...
-
REFERENCES
See March 10, 2000 Federal Register, Docket No. 00N-0553, pp. 12999-13010 - See March 10, 2000 Federal Register, Docket No. 00N-0553, pp. 12999-13010 - See NDA #020306 - Jones, S. C., A. Alavi ...
-
HOW SUPPLIED
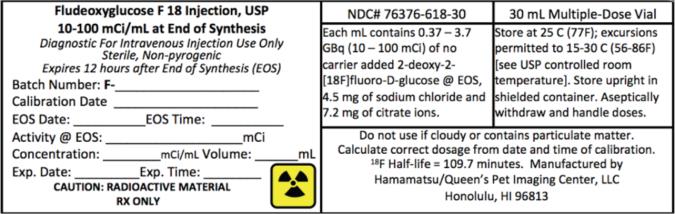
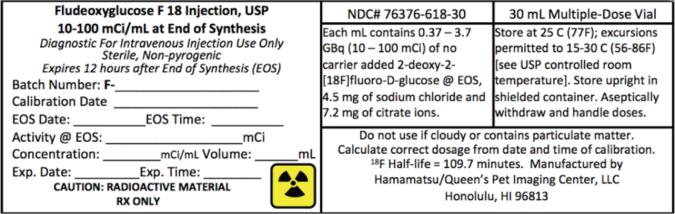
Fludeoxyglucose F 18 Injection, USP is supplied in a multi-dose septum capped 30 mL glass vial containing between 0.37 – 3.7 GBq/mL (10 - 100 mCi/mL), of no carrier added 2-deoxy-2 ...
-
STORAGE
Store Fludeoxyglucose F 18 Injection, USP at 25°C (77°F); excursions permitted to 15-30°C (59-86°F). Store Fludeoxyglucose F 18 Injection, USP multiple-dose vial upright in a lead shielded ...
-
PRINCIPAL DISPLAY PANEL
NDC# 76376-618-30 - 30 mL Multiple-Dose Vial - Fludeoxyglucose F 18 Injection, USP
-
INGREDIENTS AND APPEARANCEProduct Information