Label: ONDANSETRON HYDROCHLORIDE solution
- NDC Code(s): 68094-763-59, 68094-763-62
- Packager: Precision Dose Inc.
- This is a repackaged label.
- Source NDC Code(s): 0054-0064
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 30, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ONDANSETRON ORAL SOLUTION safely and effectively. See full prescribing information for ONDANSETRON ORAL SOLUTION. ONDANSETRON oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEOndansetron is indicated for the prevention of nausea and vomiting associated with: highly emetogenic cancer chemotherapy, including cisplatin greater than or equal to 50 mg/m2. initial and ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage - The recommended dosage regimens for adult and pediatric patients are described in Table 1 and Table 2, respectively. Corresponding doses of ondansetron tablets, orally disintegrating ...
-
3 DOSAGE FORMS AND STRENGTHSOndansetron Oral Solution, USP 4 mg/5 mL, is a clear, colorless solution.
-
4 CONTRAINDICATIONSOndansetron is contraindicated in patients: known to have hypersensitivity (e.g., anaphylaxis) to ondansetron or any of the components of the formulation [see Adverse Reactions (6.2)]. receiving ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Hypersensitivity reactions, including anaphylaxis and bronchospasm, have been reported in patients who have exhibited hypersensitivity to other selective 5-HT3 ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Hypersensitivity Reactions [see Warnings and Precautions (5.1)] QT Prolongation [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Serotonergic Drugs - Serotonin syndrome (including altered mental status, autonomic instability, and neuromuscular symptoms) has been described following the concomitant use of 5-HT3 receptor ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary: Published epidemiological studies on the association between ondansetron use and major birth defects have reported inconsistent findings and have important ...
-
9 DRUG ABUSE AND DEPENDENCEAnimal studies have shown that ondansetron is not discriminated as a benzodiazepine nor does it substitute for benzodiazepines in direct addiction studies.
-
10 OVERDOSAGEThere is no specific antidote for ondansetron overdose. Patients should be managed with appropriate supportive therapy. In addition to the adverse reactions listed above, the following adverse ...
-
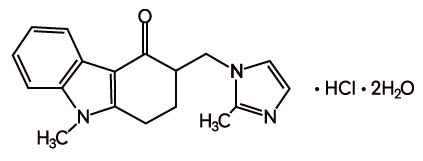
11 DESCRIPTIONThe active ingredient in ondansetron oral solution, USP is ondansetron hydrochloride, USP as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT3 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ondansetron is a selective 5-HT3 receptor antagonist. While its mechanism of action has not been fully characterized, ondansetron is not a dopamine-receptor antagonist ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenic effects were not seen in 2-year studies in rats and mice with oral ondansetron doses up to 10 mg/kg per day and 30 mg/kg ...
-
14 CLINICAL STUDIES14.1 Prevention of Chemotherapy-Induced Nausea and Vomiting - Highly Emetogenic Chemotherapy: In two randomized, double-blind, monotherapy trials, a single 24-mg oral dose of ondansetron was ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGOndansetron Oral Solution, USP - 4 mg/5 mL oral solution is supplied as a (strawberry-flavored) clear, colorless solution. NDC 68094-763-62 - 5 mL per unit dose cup - Thirty (30) cups per ...
-
17 PATIENT COUNSELING INFORMATIONHypersensitivity Reactions: Inform patients that ondansetron may cause hypersensitivity reactions, some as severe as anaphylaxis and bronchospasm. Instruct patients to immediately report any ...
-
SPL UNCLASSIFIED SECTIONPackaged by: Precision Dose, Inc. South Beloit, IL 61080 - For inquiries call Precision Dose, Inc. at 1-800-397-9228 or email druginfo@precisiondose.com - LI1762 Rev. 12/24
-
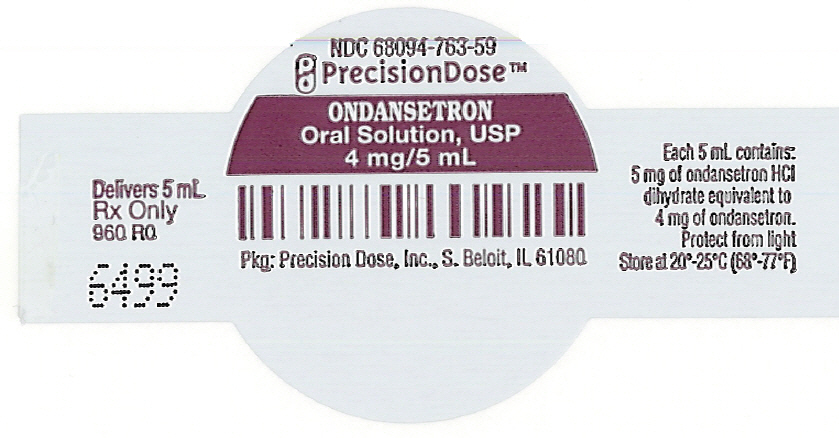
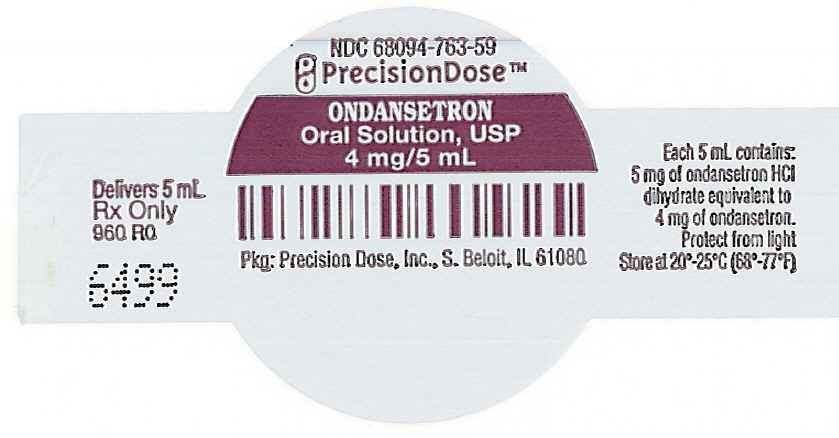
PRINCIPAL DISPLAY PANEL - 5 mL Cup LabelNDC 68094-763-59 - PrecisionDose™ ONDANSETRON - Oral Solution, USP - 4 mg/5 mL - Pkg: Precision Dose, Inc., S. Beloit, IL 61080
-
INGREDIENTS AND APPEARANCEProduct Information