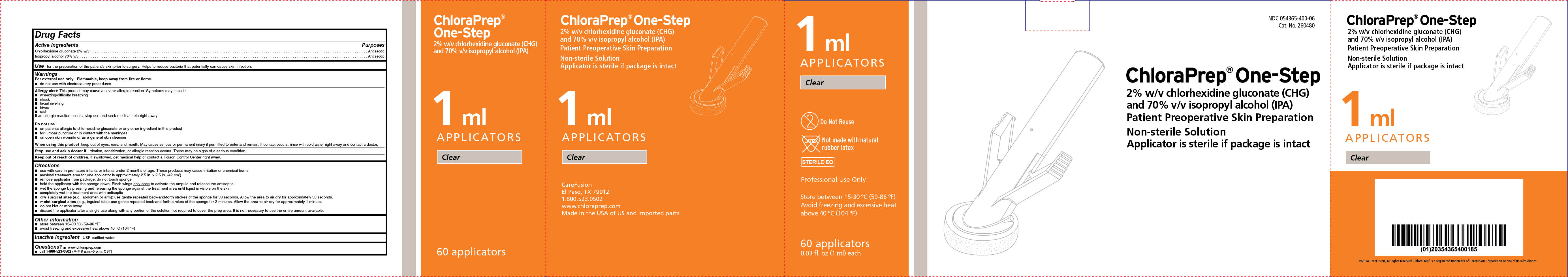
Label: CHLORAPREP ONE-STEP- chlorhexidine gluconate and isopropyl alcohol solution
- NDC Code(s): 54365-400-06
- Packager: CareFusion 213 LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purposes
- Use
-
Warnings
For external use only. Flammable, keep away from fire or flame.
- do not use with electrocautery procedures
Allergy alert:
This product may cause a severe allergic reaction. Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
Do Not Use
- on patients allergic to chlorhexidine gluconate or any other ingredient in this product.
- for lumbar puncture or in contact with the meninges
- on open skin wounds or as a general skin cleanser
When using this product
keep out of eyes, ears, and mouth. May cause serious or permanent injury if permitted to enter and remain. If contact occurs, rinse with cold water right away and contact a doctor.
-
Directions
- use with care in premature infants or in infants under 2 months of age. These products may cause irritation or chemical burns.
- maximal treatment area for one applicator is approximately 2.5 in. × 2.5 in. (42 cm 2)
- remove applicator from package; do not touch sponge
- hold the applicator with the sponge down. Pinch wings only once to activate the ampule and release the antiseptic.
- wet the sponge by pressing and releasing the sponge against the treatment area until liquid is visible on the skin
- completely wet the treatment area with antiseptic
- dry surgical sites (e.g., abdomen or arm): use gentle repeated back-and-forth strokes for approximately 30 seconds. Allow the area to air dry for approximately 30 seconds.
- moist surgical sites (e.g., inguinal fold): use gentle repeated back-and-forth strokes for approximately 2 minutes. Allow the area to air dry for approximately 1 minute.
- do not blot or wipe away
- discard the applicator after a single use along with any portion of the solution not required to cover the prep area. It is not necessary to use the entire amount available.
- Other information
- Inactive ingredients
- Questions?
-
Package/Label Principle Display Panel
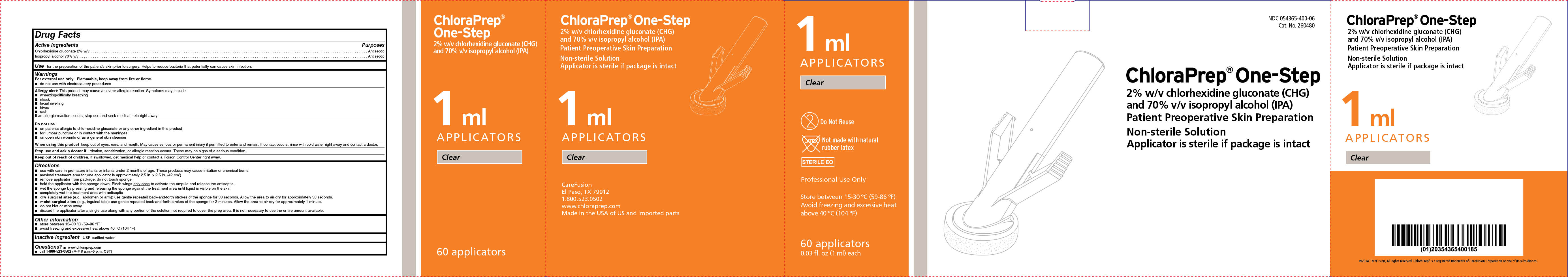
PRINCIPAL DISPLAY PANEL-CARTON
1 mL
APPLICATORS
Clear
Do Not Reuse
Not made with natural rubber latex
STERILE EO
Professional Use Only
Store between 15 - 30°C (59 - 86°F)
Avoid freezing and excessive heat
above 40°C (104°F)
60 applicators
0.03 fl. oz. (1 mL) each
NDC 054365-400-06
Cat. No. 260480
ChloraPrep ® One-Step
2% w/v chlorhexidine gluconate (CHG)
and 70% v/v isopropyl alcohol (IPA)
Patient Preoperative Skin Preparation
Non-sterile Solution
Applicator is sterile if package is intact
-
INGREDIENTS AND APPEARANCE
CHLORAPREP ONE-STEP
chlorhexidine gluconate and isopropyl alcohol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54365-400 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 20 mg in 1 mL ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54365-400-06 60 in 1 CARTON 07/23/2014 02/28/2021 1 1 in 1 POUCH 1 1 mL in 1 APPLICATOR; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020832 07/23/2014 Labeler - CareFusion 213 LLC (826496312) Registrant - Becton, Dickinson and Company (832696038) Establishment Name Address ID/FEI Business Operations CareFusion 213 LLC 826496312 analysis(54365-400) , label(54365-400) , manufacture(54365-400) , pack(54365-400) , sterilize(54365-400)