Label: PETARMOR CAPACTION- nitenpyram tablet
- NDC Code(s): 21091-141-01, 21091-142-01
- Packager: Sergeant's Pet Care Products LLC
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENTS
- WARNINGS
-
DIRECTIONS
CAPACTION Tablets kill adult fleas and are indicated for the treatment of flea infestations on dogs, puppies, cats and kittens 2 pounds of body weight or greater and 4 weeks of age and older.
A single dose of CAPACTION should kill the adult fleas on your pet. If your pet gets re-infested with fleas, you can safely give another dose as often as once per day.
To give CAPACTION Tablets, place the pill directly in your pet's mouth or hide it in food. If you hide the pill in food, watch closely to make sure your pet swallows the pill. If you are not sure that your pet swallowed the pill, it is safe to give a second pill.
Treat all infested pets in the household. Fleas can reproduce on untreated pets and allow infestations to persist.
DOSAGE
CAPACTION Tablets should be administered according to the following schedule. Weigh your pet prior to administration to ensure proper dosage. Do not administer to pets under 2 pounds.Recommended Dosage Schedule Species Body Weight Dose Nitenpyram Per Tablet Dog or Cat 2-25 lbs. One Tablet 11.4 mg Dog 25.1-125 lbs. One Tablet 57.0 mg - ADVERSE REACTIONS
-
Post-Approval Experience (Rev. 2011):
The following adverse events are based on post-approval adverse drug experience reporting. Not all adverse reactions are reported to FDA CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data. The following adverse events are listed in decreasing order of reporting frequency.
CATS: hyperactivity, panting, lethargy, itching, vocalization, vomiting, fever, decreased appetite, nervousness, diarrhea, difficulty breathing, salivation, incoordination, seizures, pupil dilation, increased heart rate, and trembling.
DOGS: lethargy/depression, vomiting, itching, decreased appetite, diarrhea, hyperactivity, incoordination, trembling, seizures, panting, allergic reactions including hives, vocalization, salivation, fever, and nervousness.
The frequency of serious signs, including neurologic signs and death, was greater in animals under 2 pounds of body weight, less than 8 weeks of age, and/or reported to be in poor body condition. In some instances, birth defects and fetal/neonatal loss were reported after treatment of pregnant and/or lactating animals.
To report adverse effects, access medical information, or obtain additional product information call 1-800-781-4738. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
For a complete listing of adverse reactions for nitenpyram reported to the CVM see:
http://www.fda.gov/AnimalVeterinary/SafetyHealth/ProductSafetyInformation
-
OTHER INFORMATION
CAPACTION Tablets begin working within 30 minutes. In studies, CAPACTION achieved greater than 90% effectiveness against adult fleas on dogs within 4 hours and cats within 6 hours.
CAPACTION Tablets are safe for pregnant or nursing dogs and cats (See Post-Approval Experience section).
When using this product, you may notice that your dog or cat will start scratching itself as fleas begin to die. The scratching behavior is temporary and is a reaction to the fleas, not the drug. In cats and dogs, this may present as transient signs of hyperactivity, panting, vocalization and excessive grooming/licking.
CAPACTION Tablets kill adult fleas that cause flea allergy dermatitis (FAD).
CAPACTION Tablets may be used together with other products, including heartworm preventives, corticosteroids, antibiotics, vaccines, de-worming medications, shampoos and other flea products.
-
FLEA INFESTATIONS ON DOGS AND CATS
In addition to the common nuisance irritations associated with infestations, fleas can be responsible for skin conditions in your pet such as flea allergy dermatitis (FAD) in the dog and miliary dermatitis in the cat. Also, fleas transmit other parasites, including tapeworms. The control of flea infestations is important to your pet's health and also reduces the problems associated with these parasites.
CAPACTION Tablets do not have an effect on fleas in the pet's environment. You may need to treat more than one time because immature fleas in and around the home will continue to develop into adults that can reinfest your pet.
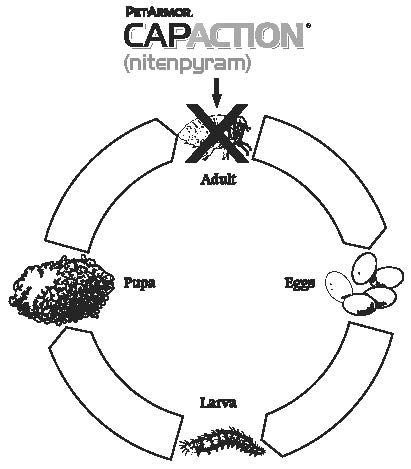
The following diagram illustrates the flea's life cycle and where CAPACTION Tablets work:
-
LIFE CYCLE OF FLEA
Fleas can be a problem because they reproduce so rapidly. A single female flea may produce up to 2,000 eggs over her lifetime. Eggs hatch and can develop into adults within only three weeks. Adult female fleas feed by ingesting blood from your pet and subsequently lay eggs, which drop off your pet's coat. Within days, larvae hatch from the eggs and live undetected in your pet's surroundings, such as the carpet, bedding and other protected areas. Flea larvae spin a cocoon, and, when appropriately stimulated, a young adult flea emerges and jumps onto your pet to continue the life cycle. When these new fleas are seen on your pet, treat with CAPACTION Tablets.
After reading this insert, if you have any questions about flea control or medical problems associated with flea infestations, consult your veterinarian, who is your pet's health care expert.
- STORAGE CONDITIONS
- G002486 PetArmor Capaction Carton Dog 2-25 lbs. 6 ct
- G002488 PetArmor Capaction Carton Cat 2-25 lbs. 6 ct
- G002487 PetArmor Capaction Carton Dog Over 25 lbs. 6 ct
-
INGREDIENTS AND APPEARANCE
PETARMOR CAPACTION
nitenpyram tabletProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:21091-141 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITENPYRAM (UNII: 3A837VZ81Y) (NITENPYRAM - UNII:3A837VZ81Y) NITENPYRAM 11.4 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (to yellow) Score no score Shape ROUND (biconvex beveled edges) Size 6mm Flavor Imprint Code RB;CG Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21091-141-01 1 in 1 CARTON 1 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141175 10/12/2021 PETARMOR CAPACTION
nitenpyram tabletProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:21091-142 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITENPYRAM (UNII: 3A837VZ81Y) (NITENPYRAM - UNII:3A837VZ81Y) NITENPYRAM 57.0 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (to yellow) Score no score Shape ROUND (biconvex beveled edges) Size 11mm Flavor Imprint Code HIH;CG Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21091-142-01 1 in 1 CARTON 1 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141175 10/12/2021 Labeler - Sergeant's Pet Care Products LLC (876995171) Establishment Name Address ID/FEI Business Operations DOTTIKON EXCLUSIVE SYNTHESIS AG 480000413 api manufacture Establishment Name Address ID/FEI Business Operations Vetio Animal Health ULC 243313714 manufacture, analysis, label, pack






