Label: AZATHIOPRINE tablet
- NDC Code(s): 68084-229-01, 68084-229-11
- Packager: American Health Packaging
- This is a repackaged label.
- Source NDC Code(s): 68382-003
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 19, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING - MALIGNANCY
Chronic immunosuppression with azathioprine, a purine antimetabolite increases risk of malignancy in humans. Reports of malignancy include post-transplant lymphoma and hepatosplenic T-cell lymphoma (HSTCL) in patients with inflammatory bowel disease. Physicians using this drug should be very familiar with this risk as well as with the mutagenic potential to both men and women and with possible hematologic toxicities. Physicians should inform patients of the risk of malignancy with azathioprine. See WARNINGS.
Close -
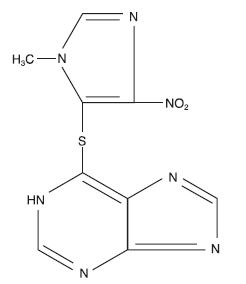
DESCRIPTIONAzathioprine is an immunosuppressive antimetabolite. Each uncoated azathioprine tablet intended for oral administration contains 25 mg or 50 mg or 75 mg or 100 mg of azathioprine. In addition ...
-
CLINICAL PHARMACOLOGYAzathioprine is well absorbed following oral administration. Maximum serum radioactivity occurs at 1 to 2 hours after oral - 35S-azathioprine and decays with a half-life of 5 hours. This is not ...
-
INDICATIONS AND USAGEAzathioprine tablets, USP are indicated as an adjunct for the prevention of rejection in renal homotransplantation. It is also indicated for the management of active rheumatoid arthritis to reduce ...
-
CONTRAINDICATIONSAzathioprine tablets should not be given to patients who have shown hypersensitivity to the drug. Azathioprine tablets should not be used for treating rheumatoid arthritis in pregnant women ...
-
WARNINGSMalignancy Patients receiving immunosuppressants, including azathioprine, are at increased risk of developing lymphoma and other malignancies, particularly of the skin. Physicians should inform ...
-
PRECAUTIONSGeneral - A gastrointestinal hypersensitivity reaction characterized by severe nausea and vomiting has been reported. These symptoms may also be accompanied by diarrhea, rash, fever, malaise ...
-
ADVERSE REACTIONSThe principal and potentially serious toxic effects of azathioprine tablets are hematologic and gastrointestinal. The risks of secondary infection and malignancy are also significant (see ...
-
OVERDOSAGEThe oral LD - 50s for single doses of azathioprine tablets in mice and rats are 2500 mg/kg and 400 mg/kg, respectively. Very large doses of this antimetabolite may lead to marrow hypoplasia ...
-
DOSAGE AND ADMINISTRATIONRenal Homotransplantation - The dose of azathioprine tablets required to prevent rejection and minimize toxicity will vary with individual patients; this necessitates careful management. The ...
-
HOW SUPPLIEDAzathioprine Tablets USP, 50 mg are yellow, round, flat, beveled edge tablets with bisect on one side; one side of the bisect is debossed with logo of "ZC" and other side is debossed with "59" and ...
-
REFERENCESClark JM. The mutagenicity of azathioprine in mice, Drosophila melanogaster, and Neurospora crassa. Mutat Res.1975; 28:87-99. Data on file, Sebela Ireland Ltd. Tagatz GE, Simmons RL ...
-
PACKAGING INFORMATIONAmerican Health Packaging unit dose blisters (see - How Supplied section) contain drug product from Zydus Pharmaceuticals (USA) Inc. as follows: (50 mg / 100 UD) NDC 68084-229-01 packaged ...
-
Package/Label Display Panel – Carton – 50mgNDC 68084- 229-01 - Azathioprine - Tablets, USP - 50 mg - 100 Tablets (10 x 10) Rx Only - Each Tablet Contains: Azathioprine ...
-
Package/Label Display Panel – Blister – 50 mgAzathioprine - Tablet, USP - 50 mg
-
INGREDIENTS AND APPEARANCEProduct Information