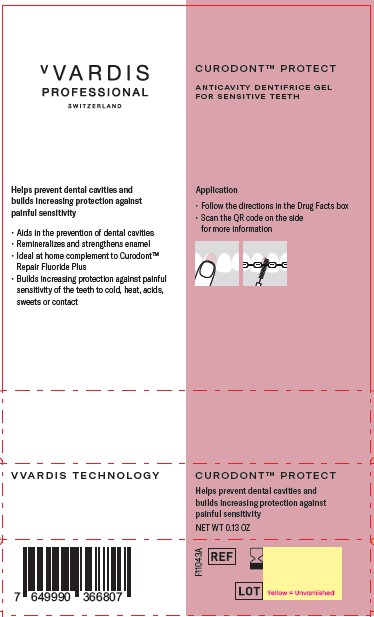
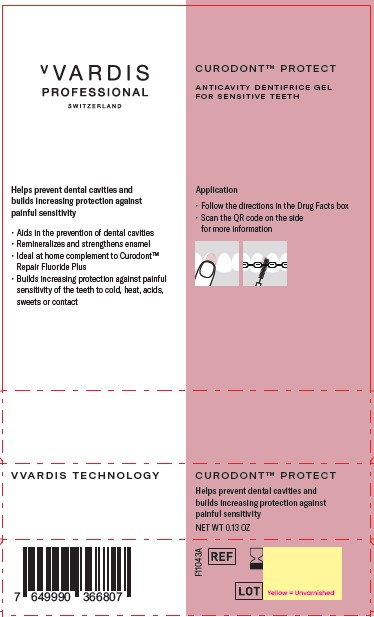
Label: CURODONT PROTECT- stannous fluoride paste, dentifrice
- NDC Code(s): 72247-107-10, 72247-107-13
- Packager: CREDENTIS AG
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
- USE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
- Adults and children 2 years of age and older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor. Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing). Supervise children as necessary until capable of using without supervision.
- Children under 2 years of age: Consult a dentist or doctor.
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CURODONT PROTECT
stannous fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72247-107 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.14 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) HYDRATED SILICA (UNII: Y6O7T4G8P9) SODIUM TRIPOLYPHOSPHATE (UNII: 5HK03SA80J) SODIUM GLUCONATE (UNII: R6Q3791S76) OLIGOPEPTIDE 104 (UNII: MUM5TLH7X6) XYLITOL (UNII: VCQ006KQ1E) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) PHOSPHORIC ACID (UNII: E4GA8884NN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72247-107-13 1 in 1 BOX 08/27/2023 1 0.13 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:72247-107-10 10 in 1 BOX 08/27/2023 2 0.13 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 08/27/2023 Labeler - CREDENTIS AG (485450345)