Label: NINLARO- ixazomib capsule
- NDC Code(s): 63020-230-01, 63020-230-02, 63020-230-03, 63020-390-01, view more
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 22, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NINLARO safely and effectively. See full prescribing information for NINLARO. NINLARO® (ixazomib) capsules, for oral use - Initial ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGENINLARO is indicated in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received at least one prior therapy. Limitations of Use ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing and Administration Guidelines - NINLARO in combination with lenalidomide and dexamethasone - The recommended starting dose of NINLARO is 4 mg administered orally once a week on ...
-
3 DOSAGE FORMS AND STRENGTHSNINLARO is available in the following capsules: 4 mg ixazomib: Light orange gelatin capsule imprinted with "Takeda" on the cap and "4 mg" on the body in black ink. 3 mg ixazomib: Light grey ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Thrombocytopenia - Thrombocytopenia has been reported with NINLARO with platelet nadirs typically occurring between Days 14-21 of each 28-day cycle and recovery to baseline by the start of ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described in detail in other sections of the prescribing information: Thrombocytopenia [see Warnings and Precautions (5.1)] Gastrointestinal Toxicities [see ...
-
7 DRUG INTERACTIONS7.1 Strong CYP3A Inducers - Avoid concomitant administration of NINLARO with strong CYP3A inducers (such as rifampin, phenytoin, carbamazepine, and St. John's Wort) [see Clinical Pharmacology ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action [see Clinical Pharmacology (12.1)] and data from animal reproduction studies, NINLARO can cause fetal harm when administered to ...
-
10 OVERDOSAGEOverdosage, including fatal overdosage, has been reported in patients taking NINLARO. Manifestations of overdosage include adverse reactions reported at the recommended dosage [see Dosage and ...
-
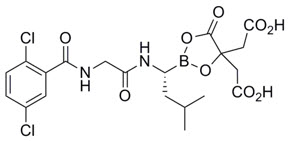
11 DESCRIPTIONIxazomib is a proteasome inhibitor. Ixazomib citrate, a prodrug, rapidly hydrolyzes under physiological conditions to its biologically active form, ixazomib. The chemical name of ixazomib citrate ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ixazomib is a reversible proteasome inhibitor. Ixazomib preferentially binds and inhibits the chymotrypsin-like activity of the beta 5 subunit of the 20S ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Ixazomib was not mutagenic in a bacterial reverse mutation assay (Ames assay). Ixazomib was considered positive in an in vitro ...
-
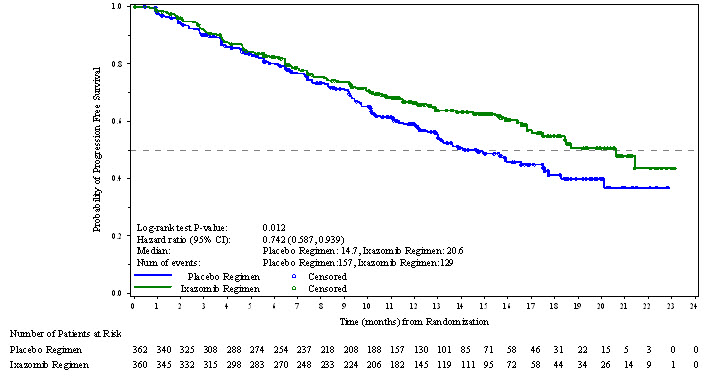
14 CLINICAL STUDIES14.1 Multiple Myeloma in Patients Who Have Received at Least One Prior Therapy - The efficacy and safety of NINLARO in combination with lenalidomide and dexamethasone was evaluated in a ...
-
15 REFERENCESOSHA Hazardous Drugs. OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - NINLARO is supplied as follows: Strength per CapsuleCapsule DescriptionOuter Carton3 Count Blister Pack1 Count Blister PackNDC - 4 mgLight orange, size 3, imprinted ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Dosing Instructions - Instruct patients to take NINLARO exactly as prescribed. Advise patients to take ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Takeda Pharmaceuticals America, Inc. Cambridge, MA 02142 - NINLARO is a registered trademark of Millennium Pharmaceuticals, Inc. ©2024 Takeda Pharmaceuticals U.S.A., Inc. All rights ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration.Revised: July/2024 - PATIENT INFORMATION - NINLARO® (nin-LAR-oh) (ixazomib) capsules - NINLARO is used ...
-
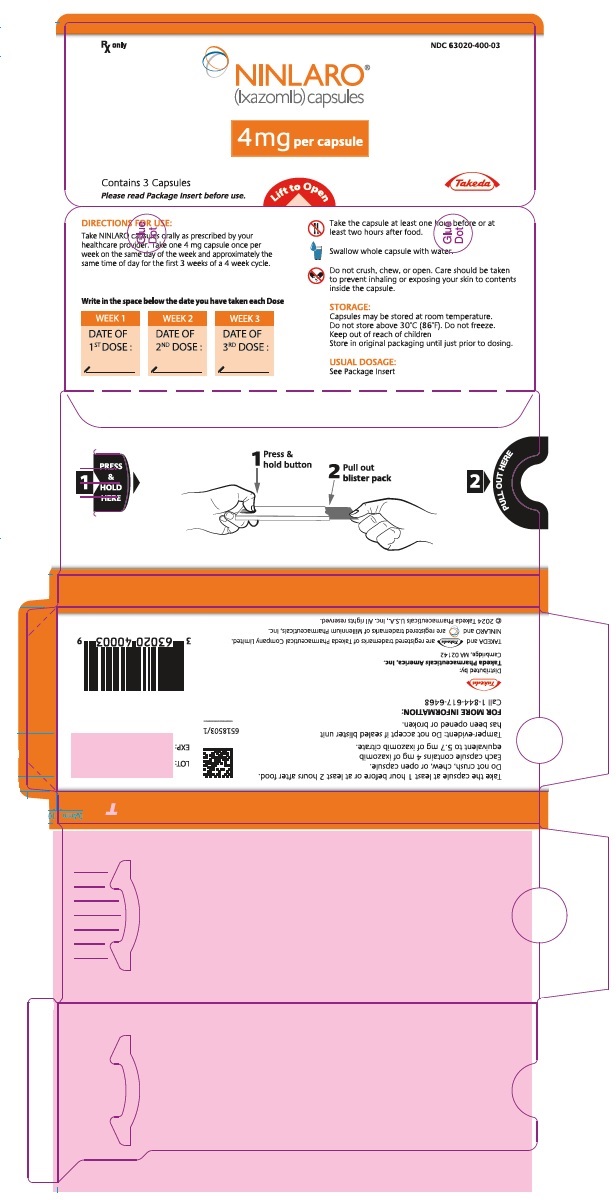
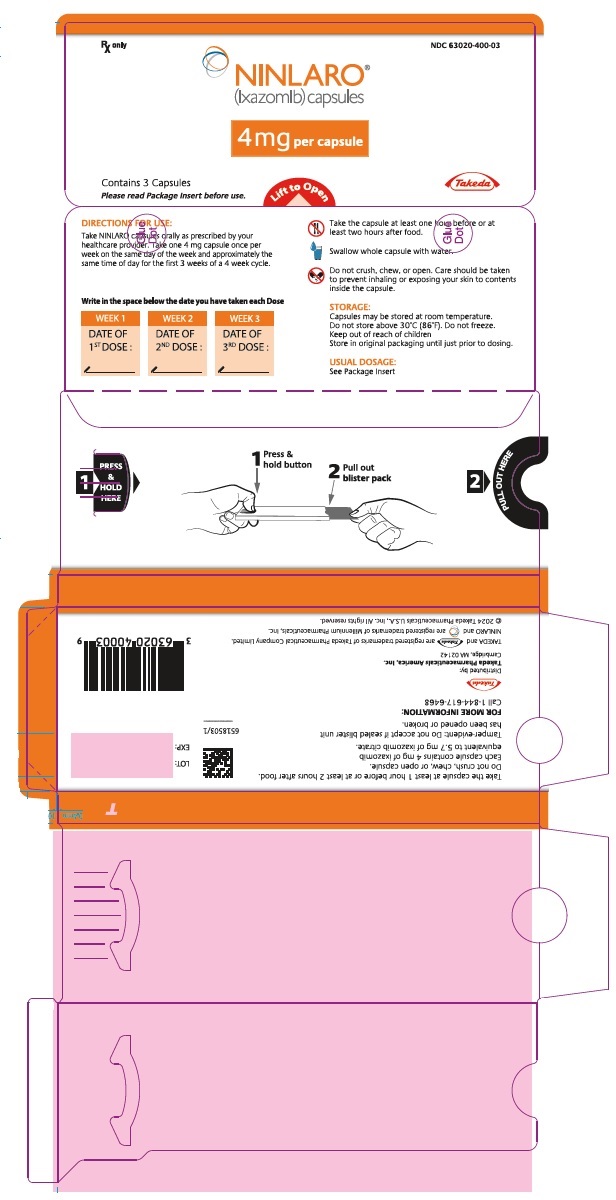
PRINCIPAL DISPLAY PANEL - 4 mg Capsule Blister PackRx only NDC 63020-400-03 - NINLARO® (ixazomib) capsules - 4 mg per capsule - Contains 3 Capsules - Please read Package Insert before use. Lift to Open - Takeda
-
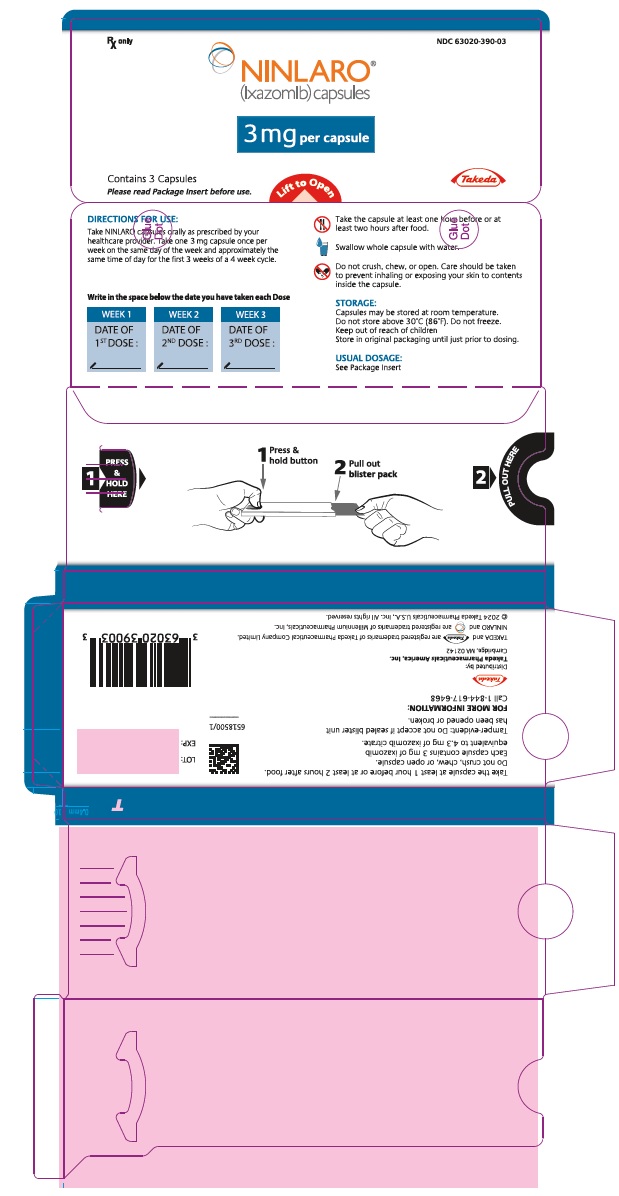
PRINCIPAL DISPLAY PANEL - 3 mg Capsule Blister PackRx only NDC 63020-390-03 - NINLARO® (ixazomib) capsules - 3 mg per capsule - Contains 3 Capsules - Please read Package Insert before use. Lift to Open - Takeda
-
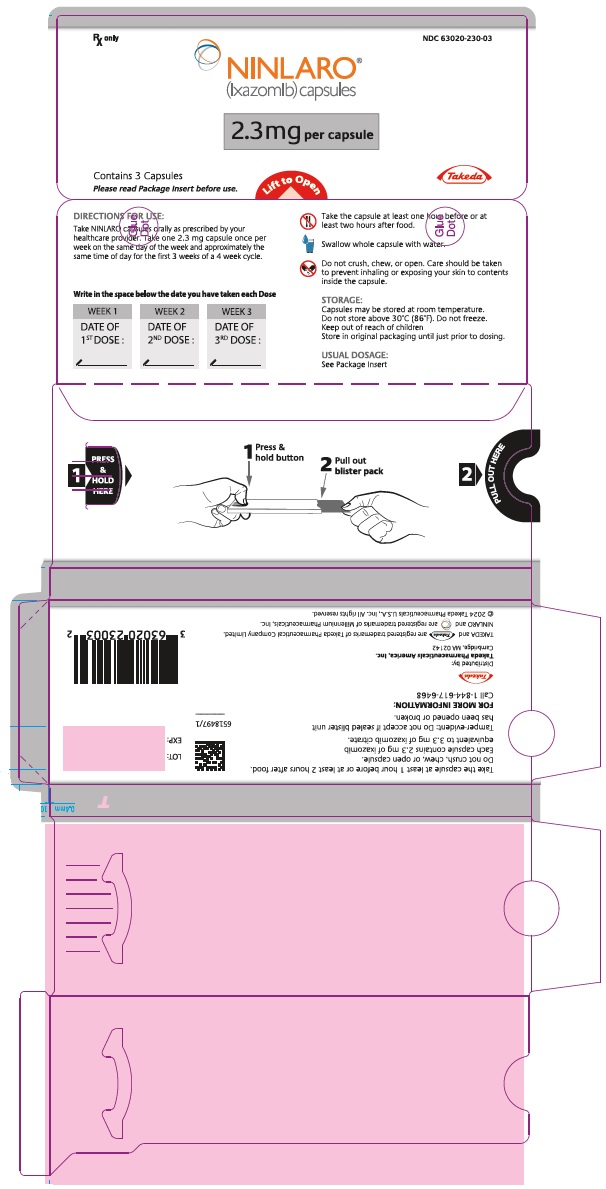
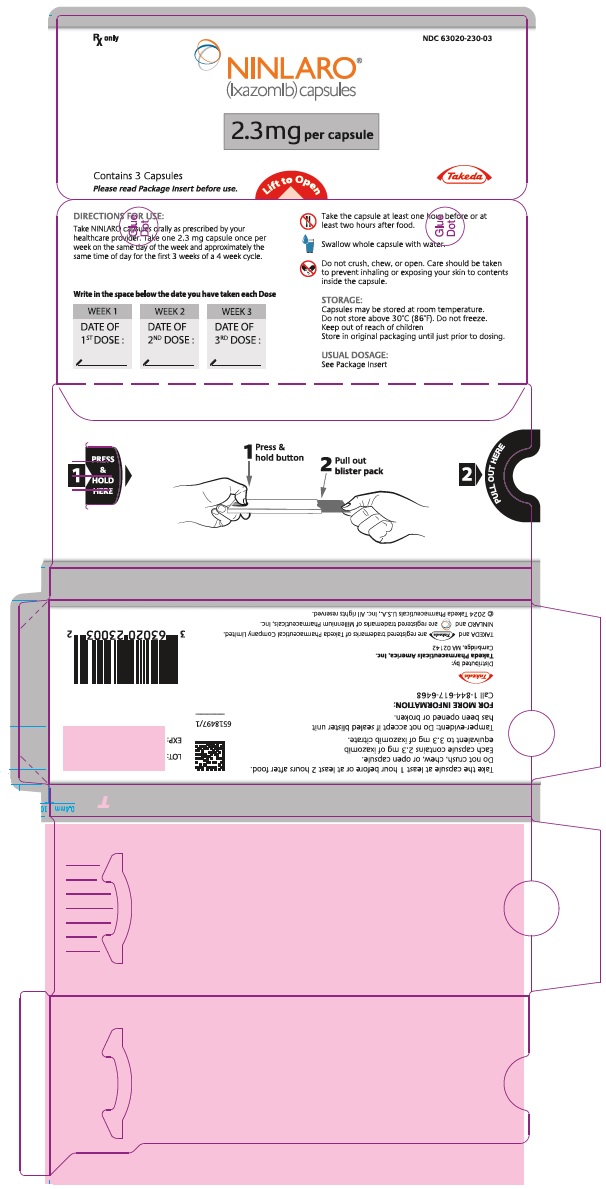
PRINCIPAL DISPLAY PANEL - 2.3 mg Capsule Blister PackRx only NDC 63020-230-03 - NINLARO® (ixazomib) capsules - 2.3 mg per capsule - Contains 3 Capsules - Please read Package Insert before use. Lift to Open - Takeda
-
INGREDIENTS AND APPEARANCEProduct Information