Label: IBUPROFEN capsule, liquid filled
- NDC Code(s): 55910-518-11, 55910-518-15, 55910-518-22, 55910-518-26
- Packager: DOLGENCORP INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
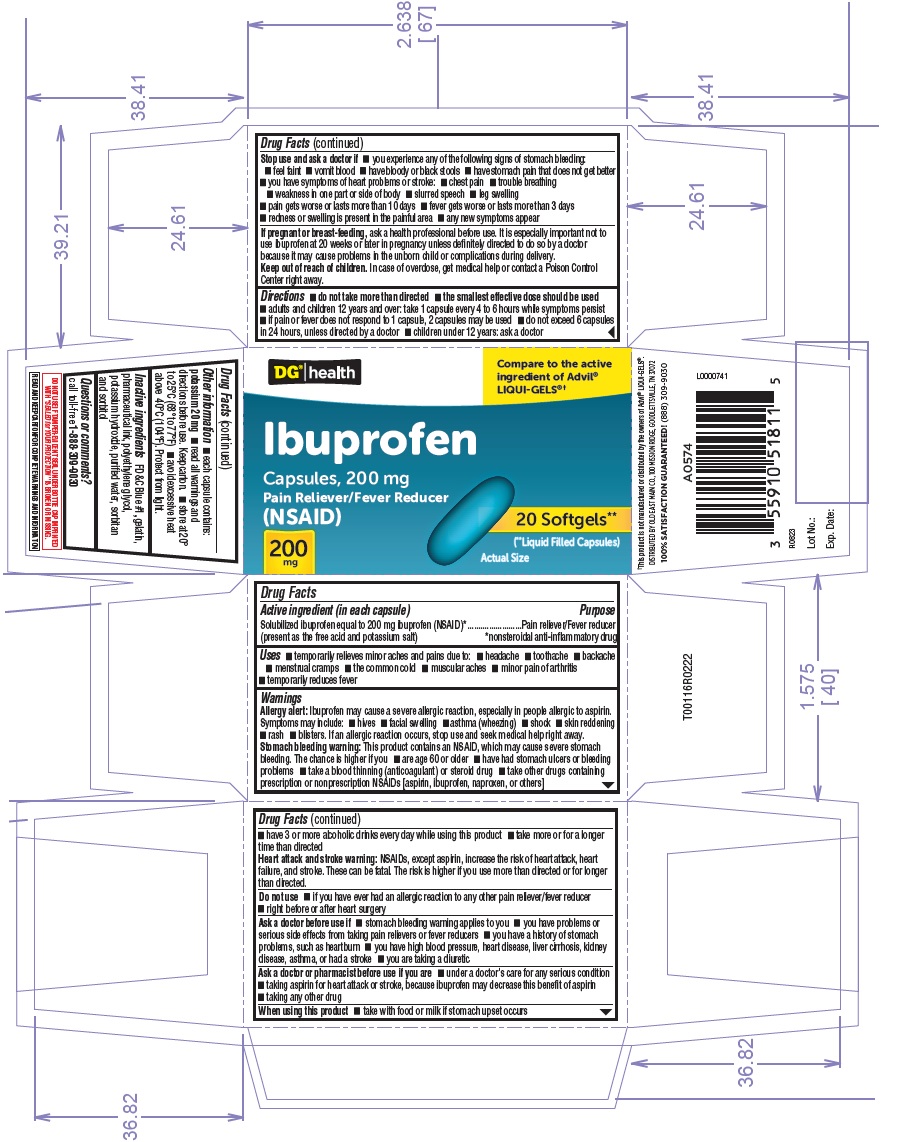
Active ingredient (in each capsule)
Solubilized ibuprofen equal to 200 mg ibuprofen (NSAID)* (present as the free acid and potassium salt)
-
Purpose
Pain reliever/Fever reducer - *nonsteroidal anti-inflammatory drug
-
Uses
temporarily relieves minor aches and pains due to: headache - toothache - backache - menstrual cramps - the common cold - muscular aches - minor pain of arthritis - temporarily reduces fever
-
Warnings
Allergy alert:Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include: hives - facial swelling - asthma (wheezing) shock - skin ...
-
Ask a doctor before use if
stomach bleeding warning applies to you - you have problems or serious side effects from taking pain relievers or fever reducers - you have a history of stomach problems, such as heartburn - you have ...
-
Ask a doctor or pharmacist before use if you are
under a doctor's care for any serious condition - taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin - taking any other drug
-
When using this product
take with food or milk if stomach upset occurs
-
Stop use and ask a doctor if
you experience any of the following signs of stomach bleeding: feel faint - vomit blood - have bloody or black stools - have stomach pain that does not get better - you have symptoms of heart problems or ...
-
If pregnant or breast-feeding,
ask a health professional before use. It is especially important not to use ibuprofen at 20 weeks or later in pregnancy unless definitely directed to do so by a doctor because it may cause ...
-
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
-
Directions
do not take more than directed - the smallest effective dose should be used - adults and children 12 years and over: take 1 capsule every 4 to 6 hours while symptoms persist - if pain or fever does ...
-
Other information
each capsule contains: potassium 20 mg - read all warnings and directions before use. Keep carton. store at 20° to 25°C (68° to 77°F) avoid excessive heat above 40°C (104°F). Protect from ...
-
Inactive ingredients
FD&C Blue #1, gelatin, pharmaceutical ink, polyethylene glycol, potassium hydroxide, purified water, sorbitan and sorbitol - For India Manufacturing Site: FD&C Blue #1, gelatin, polyethylene ...
-
Questions or comments?
call toll-free - 1-888-309-9030
-
SPL UNCLASSIFIED SECTIONDO NOT USE IF TAMPER-EVIDENT SEAL UNDER BOTTLE CAP IMPRINTED WITH “SEALED for YOUR PROTECTION” IS BROKEN OR MISSING. READ AND KEEP CARTON FOR COMPLETE WARNINGS AND INFORMATION - †This product ...
-
20's CartonDG health - Compare to the active - ingredient of Advil - ® LIQUI-GELS - ®† Ibuprofen - Capsules, 200 mg - Pain Reliever/Fever Reducer - (NSAID) 200mg - 20 ...
-
20's carton label -MADE IN INDIADG health - Compare to the active - ingredient of Advil ® LIQUI-GELS ®† Ibuprofen - Capsules, 200 mg - Pain Reliever/Fever Reducer - (NSAID) 200 mg - 20 ...
-
INGREDIENTS AND APPEARANCEProduct Information