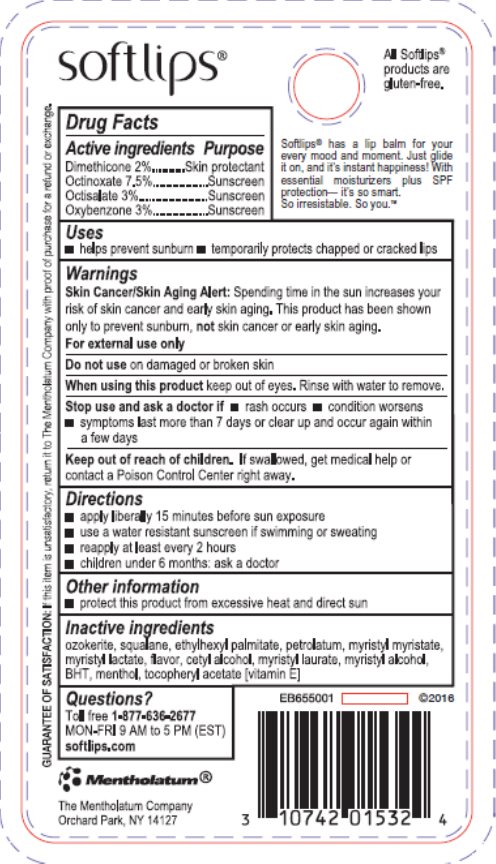
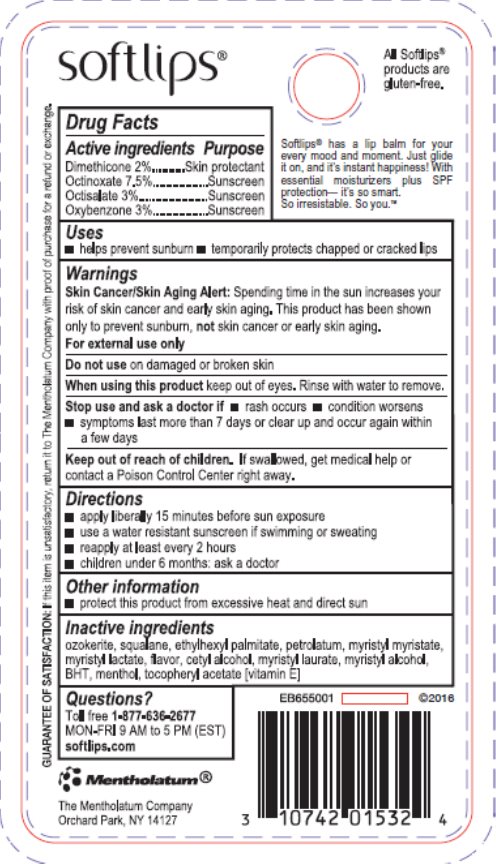
Label: SOFTLIPS WATERMELON- dimethicone, octinoxate, octisalate, oxybenzone stick
- NDC Code(s): 10742-8476-1, 10742-8476-2, 10742-8476-3
- Packager: The Mentholatum Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Uses
- Warnings
- Directions
- Inactive ingredients
- Package/Label Principal Display Panel
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SOFTLIPS WATERMELON
dimethicone, octinoxate, octisalate, oxybenzone stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10742-8476 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 20 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 30 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 30 ug in 1 g Inactive Ingredients Ingredient Name Strength SQUALANE (UNII: GW89575KF9) ETHYLHEXYL PALMITATE (UNII: 2865993309) PETROLATUM (UNII: 4T6H12BN9U) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) MYRISTYL LACTATE (UNII: 1D822OC34X) CETYL ALCOHOL (UNII: 936JST6JCN) MYRISTYL LAURATE (UNII: 58U0NZN2BT) MYRISTYL ALCOHOL (UNII: V42034O9PU) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CERESIN (UNII: Q1LS2UJO3A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10742-8476-1 1 in 1 BLISTER PACK 04/16/2012 1 2 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:10742-8476-2 2 in 1 BLISTER PACK 12/01/2016 2 2 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:10742-8476-3 3 in 1 BLISTER PACK 04/06/2020 3 2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/16/2012 Labeler - The Mentholatum Company (002105757) Registrant - The Mentholatum Company (002105757) Establishment Name Address ID/FEI Business Operations The Mentholatum Company 002105757 manufacture(10742-8476) , label(10742-8476)