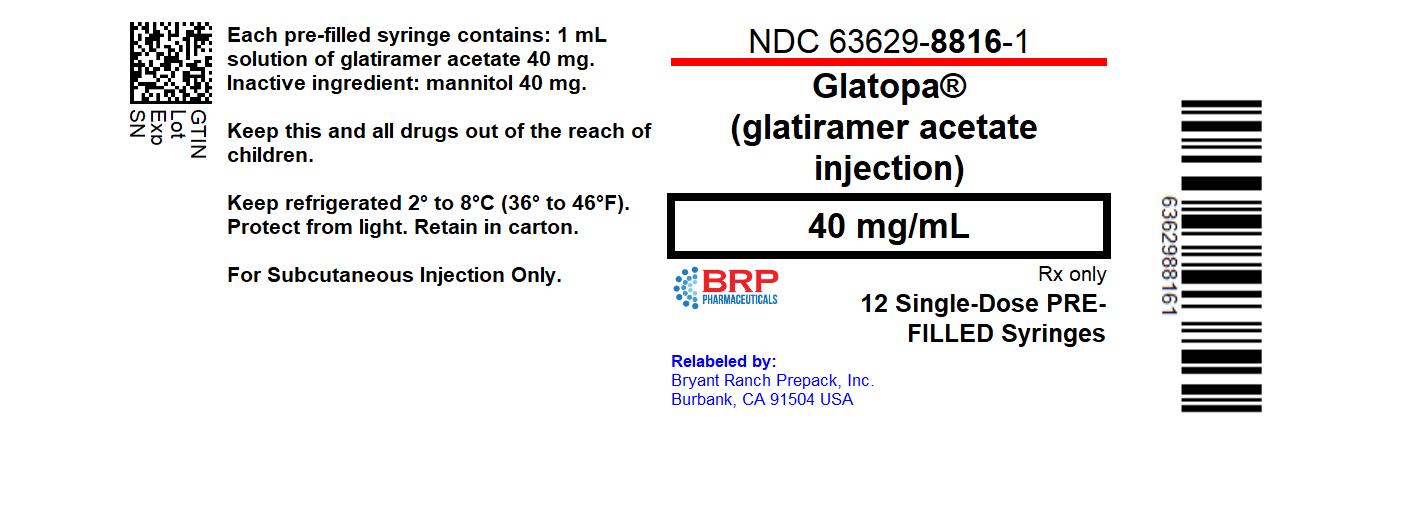
Label: GLATOPA- glatiramer acetate injection, solution
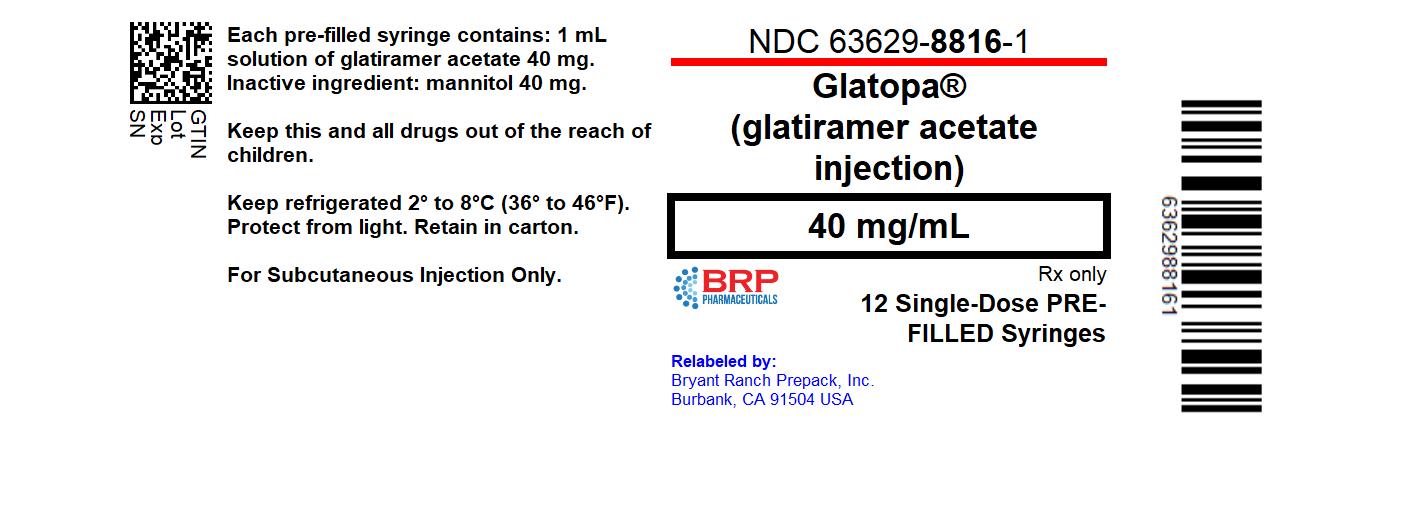
- NDC Code(s): 63629-8816-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 0781-3250
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GLATOPA® safely and effectively. See full prescribing information for GLATOPA. GLATOPA (glatiramer acetate injection), for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEGlatopa is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive ...
-
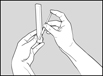
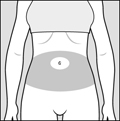
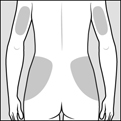
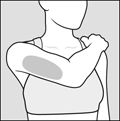
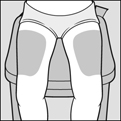
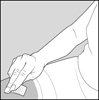
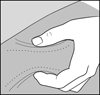
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dose - Glatopa is for subcutaneous use only. Do not administer intravenously. The dosing schedule depends on the product strength that is selected. The recommended doses ...
-
3 DOSAGE FORMS AND STRENGTHS• Injection: 20 mg per mL in a single-dose, pre-filled syringe with a white plunger. For subcutaneous use only. • Injection: 40 mg per mL in a single-dose, pre-filled syringe with a blue plunger ...
-
4 CONTRAINDICATIONSGlatopa is contraindicated in patients with known hypersensitivity to glatiramer acetate or mannitol.
-
5 WARNINGS AND PRECAUTIONS5.1 Immediate Post-Injection Reaction - Approximately 16% of patients exposed to glatiramer acetate injection 20 mg per mL in the five placebo-controlled trials compared to 4% of those on ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described elsewhere in the labeling: • Immediate Post-Injection Reaction [see Warnings and Precautions (5.1)] • Chest Pain [see Warnings and ...
-
7 DRUG INTERACTIONSInteractions between glatiramer acetate injection and other drugs have not been fully evaluated. Results from existing clinical trials do not suggest any significant interactions of glatiramer ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available human data on the use of Glatopa in pregnant women are not sufficient to support conclusions about drug-associated risk for major birth defects and ...
-
11 DESCRIPTIONGlatiramer acetate, the active ingredient of Glatopa, consists of the acetate salts of synthetic polypeptides, containing four naturally occurring amino acids: L-glutamic acid, L-alanine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism(s) by which glatiramer acetate exerts its effects in patients with MS are not fully understood. However, glatiramer acetate is thought to act by modifying ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a 2-year carcinogenicity study, mice were administered up to 60 mg/kg/day glatiramer acetate by subcutaneous ...
-
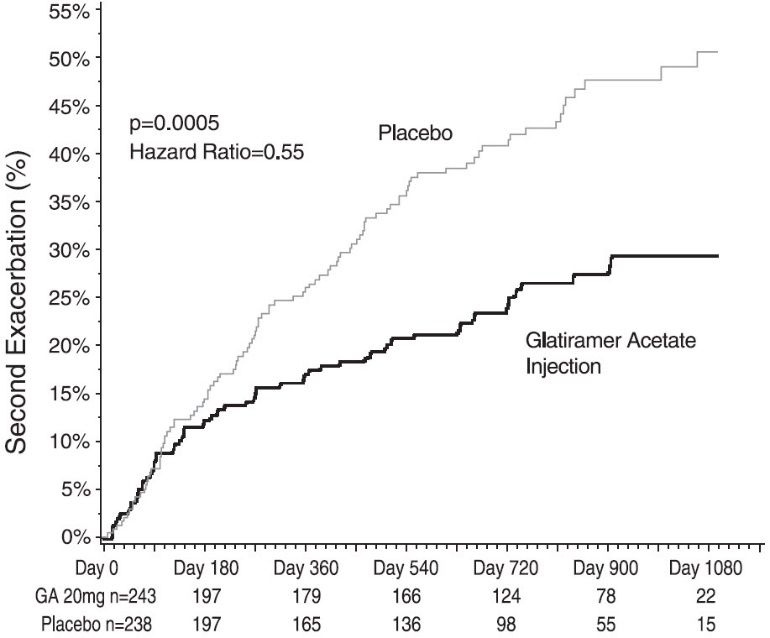
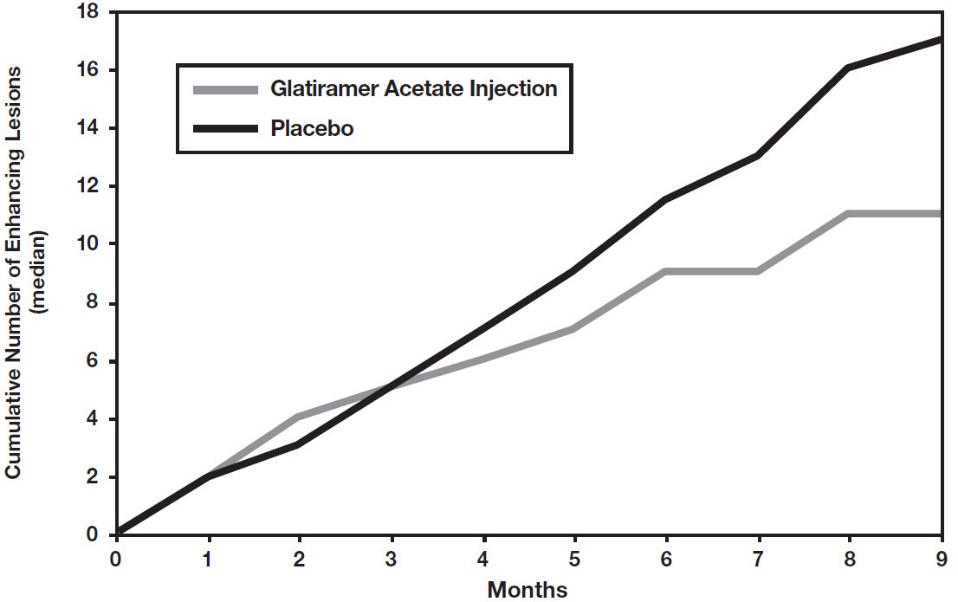
14 CLINICAL STUDIESEvidence supporting the effectiveness of glatiramer acetate injection derives from five placebo-controlled trials, four of which used a glatiramer acetate injection dose of 20 mg per mL per day ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGGlatopa (glatiramer acetate injection) is a clear, colorless to slightly yellow, sterile, nonpyrogenic solution in a 1 mL single-dose glass syringe with attached 1/2 inch length, 29 gauge needle ...
-
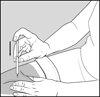
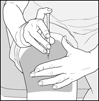
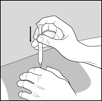
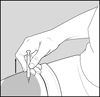
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Immediate Post-Injection Reaction - Advise patients that Glatopa may cause various ...
-
PATIENT INFORMATIONGlatopa® (gluh-TOH-puh) (glatiramer acetate injection) for Subcutaneous Use - Read this Patient Information before you start using Glatopa and each time you get a refill. There may be new ...
-
PRINCIPAL DISPLAY PANELGlatiramer Acetate Injection Solution #12
-
INGREDIENTS AND APPEARANCEProduct Information