Label: PREDNISONE tablet
- NDC Code(s): 70518-0307-0
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 0603-5338
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
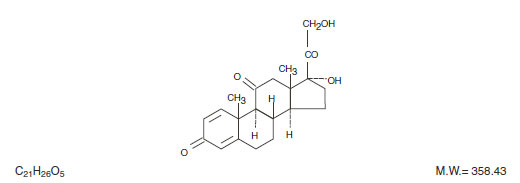
DESCRIPTIONPredniSONE Tablets contain prednisone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the ...
-
CLINICAL PHARMACOLOGYNaturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic ...
-
INDICATIONS AND USAGEPredniSONE Tablets are indicated in the following conditions: 1. Endocrine Disorders - Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the first choice; synthetic ...
-
CONTRAINDICATIONSPrednisone tablets are contraindicated in systemic fungal infections and known hypersensitivity to components.
-
WARNINGSGeneral - Rare instances of anaphylactoid reactions have occurred in patients receiving corticosteroid therapy (see - ADVERSE REACTIONS: Allergic Reactions). Increased dosage of rapidly ...
-
PRECAUTIONSGeneral Precautions - The lowest possible dose of corticosteroids should be used to control the condition under treatment. When reduction in dosage is possible, the reduction should be ...
-
ADVERSE REACTIONS(listed alphabetically, under each subsection) The following adverse reactions have been reported with prednisone or other corticosteroids: Allergic Reactions - anaphylactoid or ...
-
DOSAGE AND ADMINISTRATIONGastric irritation may be reduced if taken before, during, or immediately after meals or with food or milk. The maximal activity of the adrenal cortex is between 2 am and 8 am, and it is minimal ...
-
HOW SUPPLIEDPredniSONE Tablets are available in the following strengths and package sizes: 10 mg (white, round, scored, debossed “5093” on one side and debossed “V” on the reverse side) NDC ...
-
REFERENCESFekety R. Infections associated with corticosteroids and immunosuppressive therapy. In: Gorbach SL, Bartlett JG, Blacklow NR, eds. Infectious Diseases. Philadelphia: WBSaunders Company ...
-
SPL UNCLASSIFIED SECTIONRepackaged and Distributed By: Remedy Repack, Inc. 625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762
-
PRINCIPAL DISPLAY PANELDRUG: Prednisone - GENERIC: Prednisone - DOSAGE: TABLET - ADMINSTRATION: ORAL - NDC: 70518-0307-0 - COLOR: white - SHAPE: ROUND - SCORE: Two even pieces - SIZE: 9 mm - IMPRINT: 5093;V - PACKAGING: 21 in 1 BOX ...
-
INGREDIENTS AND APPEARANCEProduct Information